From Lab to Scale: Transforming Industrial Off-Gases into Bio-Wealth
Published in Chemistry

The effort to reduce carbon emissions in industries, especially in steel manufacturing, is filled with challenges. The steel industry, a major source of CO2 emissions, urgently needs sustainable solutions. Our research focuses on turning industrial off-gases into formate through enzymatic conversion, showing promise in tackling this issue1,2.
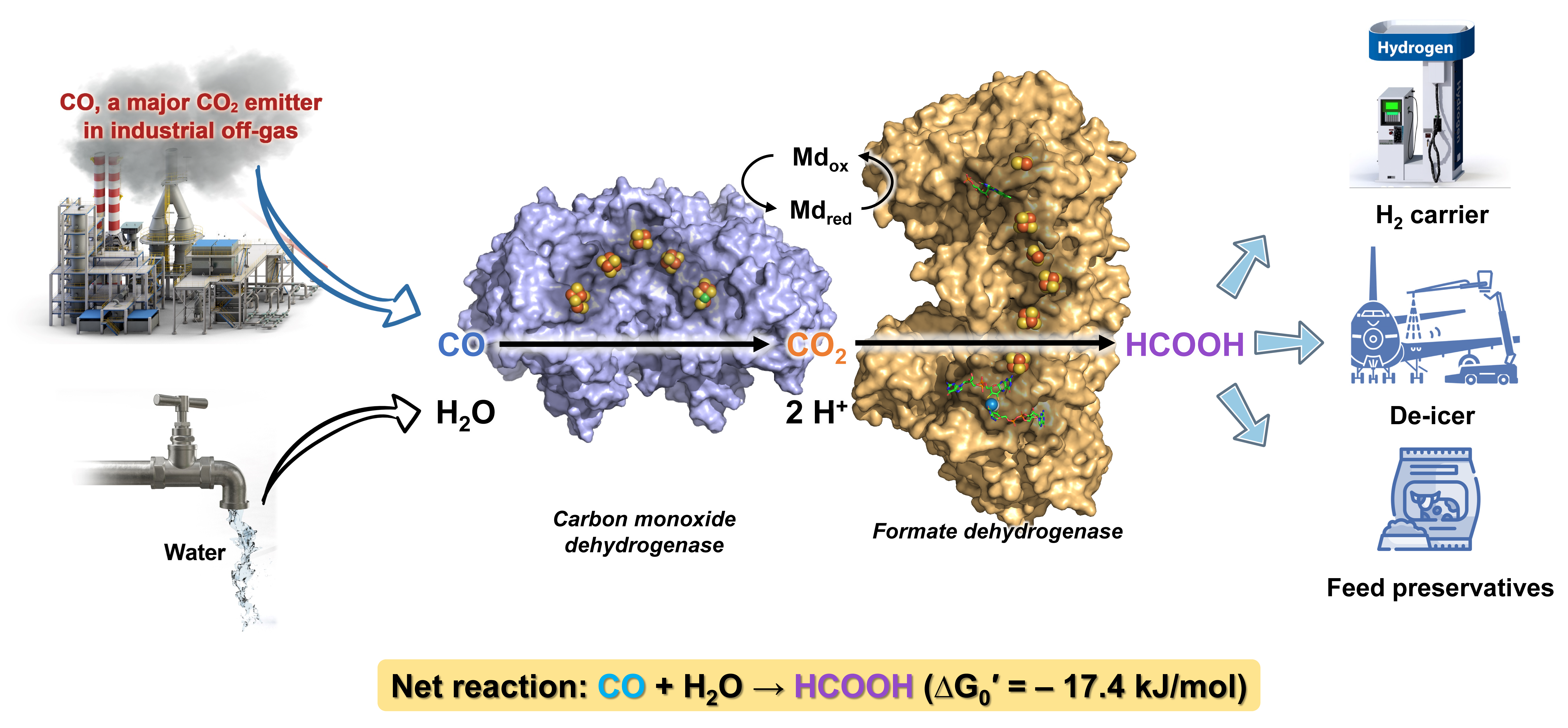
Our project began with the goal of reducing the steel industry's CO emissions. Traditional methods burn CO, releasing CO2 and missing the chance to use CO as a resource for producing chemicals. We drew inspiration from nature's carbon-fixing Wood‒Ljungdahl pathway, aiming to replicate it industrially to convert CO into formate, a versatile chemical used in various applications3,4. We used CO dehydrogenase1 and formate dehydrogenase4 enzymes to transform CO into formate efficiently (Figure 1). This process, called enzymatic CO hydration (enCOH), operates under mild conditions, avoiding the energy-intensive and harsh processes typically used, and offering a sustainable, resource-effective alternative.
Our main challenge was to develop an enzyme system that could effectively process industrial off-gases, which contain a mix of gases and impurities. Finding and optimizing enzymes that remain active under such conditions was crucial5. Through tests, we found enzymes that work efficiently despite the contaminants and varying gas ratios in steel mill off-gases. Another hurdle was scaling up our lab process to a larger, semi-pilot scale for industrial use. We needed a system that could handle varying off-gas compositions, maintain enzyme activity for long periods, and produce formate in industrially useful concentrations.
Our results show that it's feasible to use enzymatic reactions to turn industrial off-gases into valuable chemicals, offering the steel industry a feasible way to lessen its carbon footprint. The process not only utilizes CO emissions but also produces formate, useful in many applications. The next steps involve scaling up and assessing the economic practicality of this technology in industrial settings. A successful pilot at a steel mill shows its potential for wider use, possibly extending to other industries with similar emissions. Further enhancements and integration of this technology could aid global carbon neutrality efforts.
In summary, our work demonstrates the potential of enzyme engineering and biocatalysis in addressing environmental issues. By replicating nature's methods of carbon fixation, we've demonstrated paths for industrial decarbonization and sustainable chemical production, aiming for a greener future for high-emission industries.
References:
- Kim, S.M. et al. O2-tolerant CO dehydrogenase via tunnel redesign for the removal of CO from industrial flue gas. Nat. Catal. 5, 807‒817 (2022). https://doi.org/10.1038/s41929-022-00834-y
- Kim, S.M. et al. Identifying a key spot for electron mediator-interaction to tailor CO dehydrogenase’s affinity. Nat. Commun., 15, 2732 (2024). https://doi.org/10.1038/s41467-024-46909-1
- Hwang, H.W. et al. Two-stage bioconversion of carbon monoxide to biopolymers via formate as an intermediate. Chem. Eng. J. 389, 124394 (2020). https://doi.org/10.1016/j.cej.2020.124394
- Jang, J., Jeon, B.W. & Kim, Y.H. Bioelectrochemical conversion of CO2 to value-added product formate using engineered Methylobacterium extorquens. Sci. Rep. 8, 7211 (2018). https://doi.org/10.1038/s41598-018-23924-z
- Kim, S.M., Kang, S.H., Jeon, B.W. & Kim, Y.H. Tunnel engineering of gas-converting enzymes for inhibitor retardation and substrate acceleration. Bioresour. Technol. 394, 130248 (2024). https://doi.org/10.1016/j.biortech.2023.130248
*Image credit for poster: Pixabay.
Follow the Topic
-
Nature Chemical Engineering

This is a new monthly online journal dedicated to publishing the most significant original research, commentary and analysis of direct relevance to the diverse community of chemical engineers.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in