Gas-Phase Synthesis of the C40 Nano Bowl
Published in Chemistry

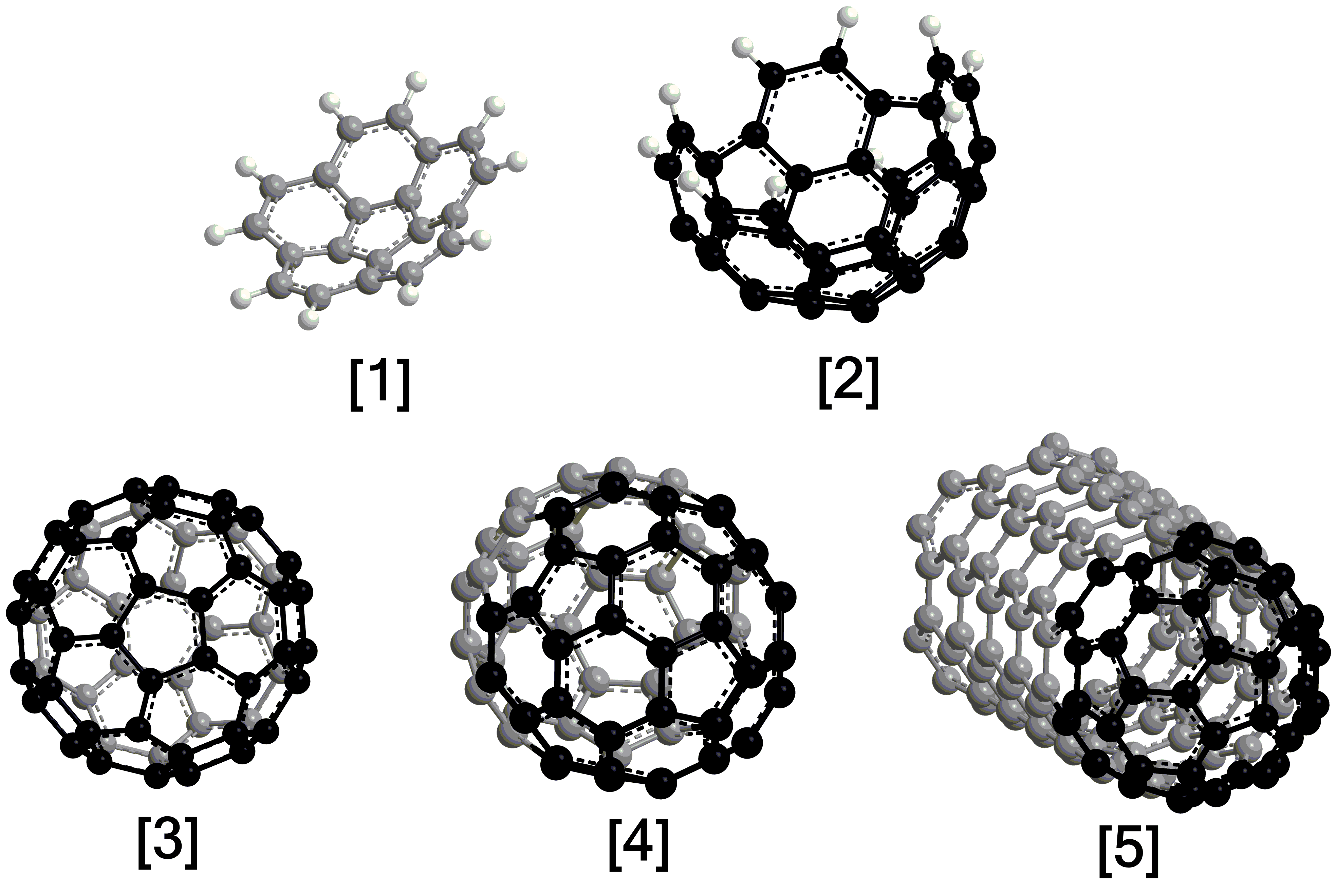
Nanobowls, which belong to an extended family of curved (three-dimensional) polycyclic aromatic hydrocarbons (PAHs) with the prototype species being the smallest bowl-shaped corannulene (C20H10) molecule, represent vital molecular building blocks of fullerenes and nanotubes as detected in combustion systems and in deep space (Fig. 1). However, synthesis of nanobowls more complex than corannulene, such as C40 (C40H10) and C50 (C50H10), have remained a fundamental challenge. Whereas the formation and isolation of these nanobowls is interesting by itself from the synthetic chemistry viewpoint, the ultimate goal is to exploit these nanostructures as transitional templates to direct the synthesis of the closed icosahedral C60–fullerene structure and to employ them as end-caps of nanotubes. While there have been synthetic routes preparing mono- to pentabenzocorannulenes (C40H20), they do not rationalize the pathways to naturally prepared fullerenes detected in combustion flames, in meteorites such as Allende and Murchison, and in the planetary nebula TC-1. Consequently, hitherto elusive high temperature chemical routes for the synthesis of fullerenes along with their nanobowls must exist either from the bottom up or the top down.
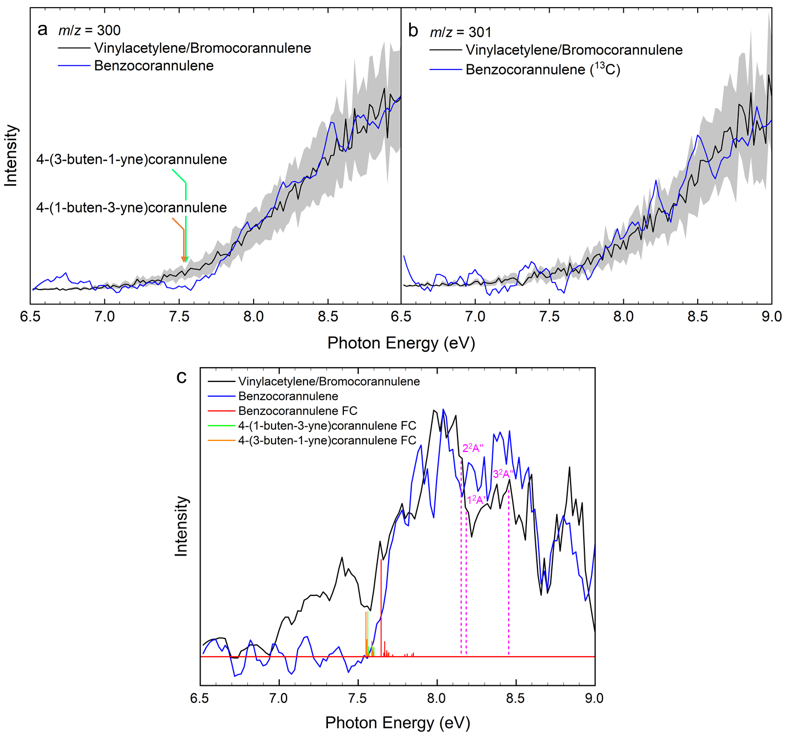
By merging molecular beam experiments with electronic structure calculations, we reveal a complex chain of reactions initiated through the gas-phase preparation of benzocorannulene (C24H12) via ring annulation of the corannulenyl radical (C20H9•) by vinylacetylene (C4H4). The experiments were conducted at the Swiss Light Source (SLS) utilizing a chemical microreactor coupled to a molecular beam apparatus operated with a double velocity map imaging (VMI) photoelectron photoion coincidence (PEPICO) spectrometer. Combining this setup with incident vacuum ultraviolet (VUV) synchrotron radiation, we obtained a product molecular formula (C24H12) using mass spectrometry and verified the benzocorannulene structural isomer via photoionization efficiency curves (PIEs) and photoion mass-selected threshold photoelectron spectra (ms-TPES) (Fig. 2).
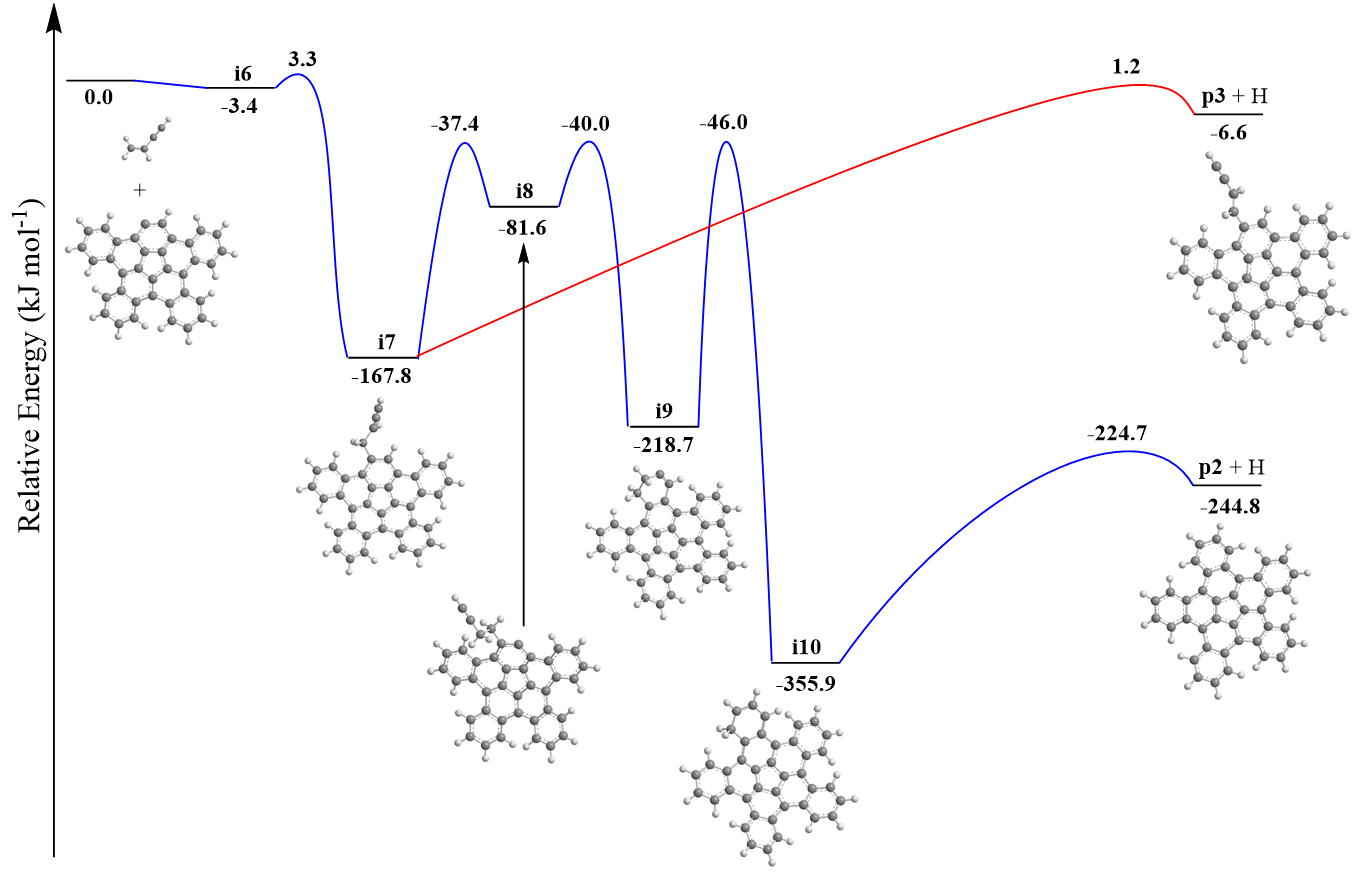
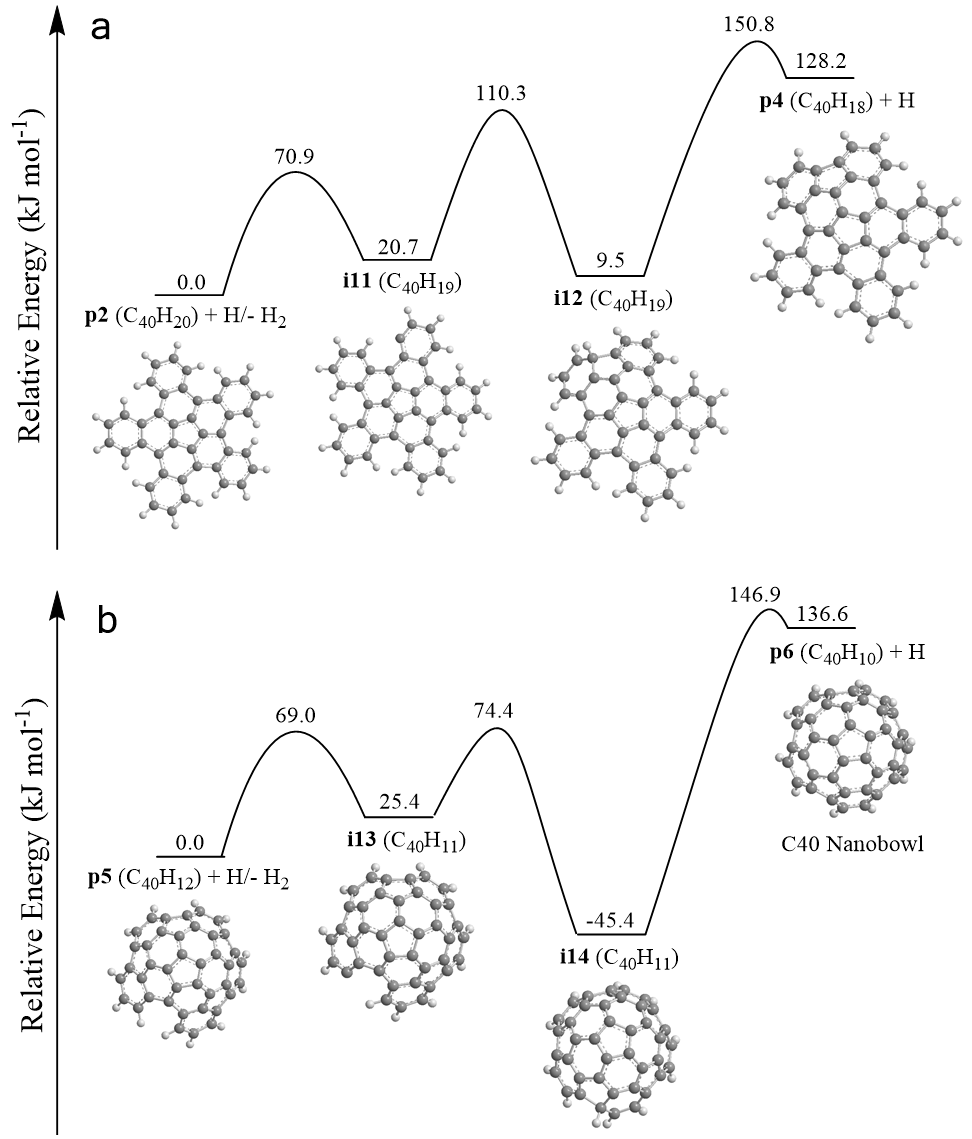
Exploiting benzocorannulene (C24H12) as a benchmark, we further expose in silico that the benzannulation mechanism can be expanded up to pentabenzocorannulene (C40H20) followed by successive cyclodehydrogenation to the C40 nanobowl (C40H10) (Figs. 3 and 4). The exploitation of linearly scaling coupled cluster method for electronic structure calculations of molecules as large as C40H20 opens a previously unavailable avenue for accurate exploration of potential energy diagrams of complex molecules with chemical accuracy allowing to obtain detailed information on energies of metastable states/products, barriers, and chemical pathways complementary to experimental data. This high-temperature route opens up a facile, isomer-selective bottom up pathway to nanobowls via resonantly stabilized free-radical intermediates and ring annulation in combustion flames and in planetary nebulae, which may act as precursors to buckminsterfullerene (C60) thus changing our conception of the formation of complex carbon nanostructures in our Galaxy.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in