Giant centipedes from the Australian outback use different venom cocktails for predation and defense
Published in Ecology & Evolution, Protocols & Methods, and Zoology & Veterinary Science

It all started in 2018 when I moved to Australia to do a PhD on centipede venom glands. At that time venom modulation was sort of a hot topic as several papers had been published showing that some venomous animals—including cone snails
I hadn’t worked with venoms before and very quickly realized how diverse they are. Venoms have evolved more than 100 times in all kinds of different animals
My species of interest, the red-headed centipede Scolopendra morsitans, lives in the Australian outback. To collect this species we would drive five hours inland from Brisbane to a small town named Glenmorgan; equipped with long forceps, lots of plastic containers, coffee, bread, avocado, tuna, killer python lollies and several cartons of beer. We usually spent the days walking around flipping rocks and dead wood, but we found that one of the best spots to find centipedes is the local rubbish tip (Fig.1 B). There are lots of places at the tip where centipedes (and other animals) can hide from the burning sun: underneath plastic bags, cardboard, old mattresses, etc. We also drove around at night in hope to spot some cool animals on the roads (Fig. 1 C); during the night it can get very cold in the outback so some animals get attracted by asphalted roads which are still hot from the heat of the day. We saw snakes and centipedes but also echidnas, geckos, dingoes, owls, spiders, frogs and lots and lots of “stick snakes”—sticks and twigs on the road that made us think they were snakes or centipedes. We usually stayed two to three days and collected 5–35 centipedes per trip.

Even though centipedes have been roaming this planet for over 400 million years, not many people have studied centipedes and their venom. One reason for this might be that their venoms can’t kill you—but it hurts a lot when they sting you! (Yes they technically sting and don’t bite, as their venom claws are not part of the mouth parts but modified legs.) They use their venom not only for defense but also for predation. Centipedes eat pretty much anything: Crickets, spiders, lizards, snakes, worms and even small mammals which are much bigger than the centipede itself. This is only possible because of their venom, which can quickly paralyze their prey. As predatory and defensive venoms should contain different acting toxins—paralyzing toxins for predation vs. pain-causing toxins for defense—we wanted to know more about whether centipedes always use the same venom cocktail or whether they can somehow adjust venom composition depending on whether they use it for predation or defense.
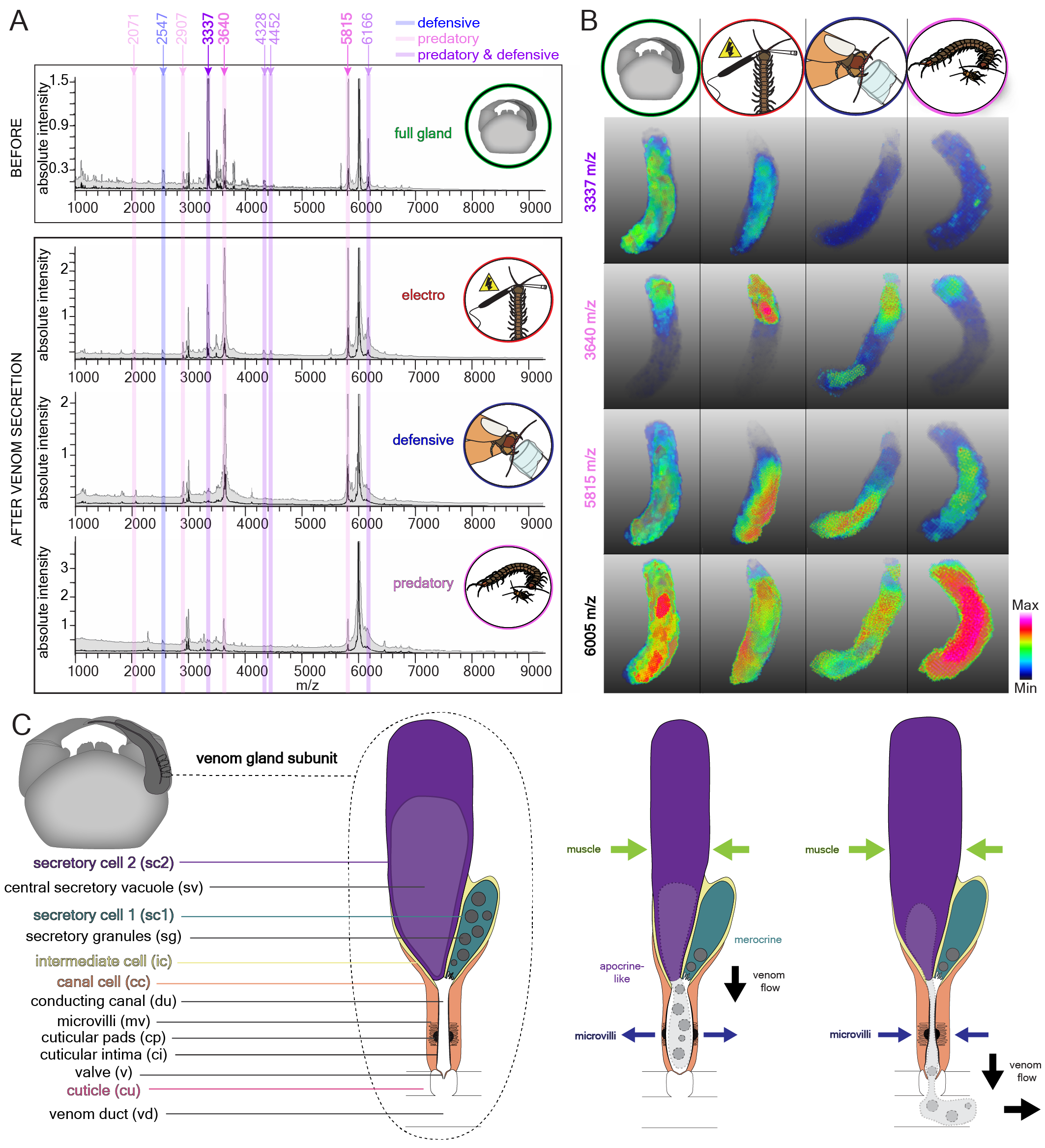
Because it is difficult (or maybe even impossible) to collect true predatory venom, we used 3D Mass Spectrometry Imaging (MSI) to look at centipede venom glands (and the toxins they contain) before and after venom was used in different contexts. MSI is a way to map the distribution of molecules across tissue sections without the need to use specific stains. The idea behind it is that venom glands from before venom was secreted should contain the whole venom repertoire, while venom glands after the centipede attacked and injected venom into a cricket should lack toxins that are used in a predatory context, and venom glands after defensive stinging should lack toxins that are used in a defensive context. In 2022, we submitted a manuscript to Nature Ecology & Evolution showing that S. morsitans uses different venom cocktails for predation and defense (using 3D MSI, immunohistochemistry and a combination of proteomics and transcriptomics). At that time, I had also just submitted my PhD thesis and was naïve enough to think my work on that project was done… The reviewers suggested that we should include more statistical analyses and it would be nice to have a mechanism of secretion that would explain how centipedes control venom composition. By the time we got that feedback I had just started two new jobs and had no idea when and how I was supposed to do any additional work on that manuscript. Long story short, two years after the initial submission and many experiments later (now also including serial block-face electron microscopy), our centipede venom modulation story is finally complete! I think the whole team is very glad that we took the time and effort to include more statistical analyses and, most importantly, find a way to understand HOW S. morsitans modulates venom composition: it is a combination of uneven toxin distribution inside the venom gland and a dual mechanism of secretion which involves neuro-muscular innervation as well as innervation via neurotransmitters or hormones (Fig. 3). Furthermore, we use venom modulation as an example to show how different aspects of venom biology—evolution, morphology, ecology and pharmacology—are interlinked: Pre-existing structures and tissues (e.g. front legs with epidermal glands) shape the morphology of the evolved venom apparatus (e.g. forcipule with internalized venom gland), while venom gland morphology determines whether an animal is able to modulate venom secretion (e.g. are there spatially separated gland lumens for storing different toxins), which influences venom composition, and the intended function of a venom (e.g. predation vs. defense) defines the pharmacological properties of toxins within the venom (e.g. paralyzing or pain-causing). While many questions about the ecology and evolution of venom remain, these results allowed me to answer two of the key questions that I had when I started my work on centipede venom, namely how venom production and injection has evolved and what some of the ecological functions of venom are.
References:
1. Dutertre S, Jin AH, Vetter I, Hamilton B, Sunagar K, Lavergne V, et al. Evolution of separate predation-and defence-evoked venoms in carnivorous cone snails. Nat Commun. 2014;5:1–9.
2. Walker AA, Mayhew M, Jin J, Herzig V, Undheim EAB, Sombke A, et al. The assassin bug Pristhesancus plagipennis produces distinct venoms in separate gland lumens. Nat Commun. 2018;9:755.
3. Inceoglu B, Lango J, Jing J, Chen L, Doymaz F, Pessah IN, et al. One scorpion, two venoms: Prevenom of Parabuthus transvaalicus acts as an alternative type of venom with distinct mechanism of action. Proc Natl Acad Sci U S A. 2003;100:922–7.
4. Wigger E, Kuhn-Nentwig L, Nentwig W. The venom optimisation hypothesis: a spider injects large venom quantities only into difficult prey types. Toxicon. 2002;40:749–52.
5. Morgenstern D, King GF. The venom optimization hypothesis revisited. Toxicon. 2013;63:120–8.
6. Schendel V, Rash LD, Jenner RA, Undheim EAB. The diversity of venom: The importance of behavior and venom system morphology in understanding its ecology and evolution. Toxins (Basel). 2019;11:666.
Follow the Topic
-
Nature Ecology & Evolution

This journal is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Biodiversity and ecosystem functioning of global peatlands
Publishing Model: Hybrid
Deadline: Jul 27, 2026
Understanding species redistributions under global climate change
Publishing Model: Hybrid
Deadline: Jun 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in