The search for body fluid biomarkers for different neurological diseases such as Alzheimer’s Disease (AD), Dementia with Lewy Bodies (DLB), Multiple Sclerosis (MS), and Parkinson Disease (PD) has a long history. So far, good biomarkers were found, however, we are still aiming to identify novel biomarkers specifically for early diagnosis, optimal stratification of patients in clinical trials or to monitor disease progression. One of the main sources for the identification of such novel biomarkers is the cerebrospinal fluid (CSF) since it is in direct contact with the central nervous system and thus reflects the pathophysiological processes. By analyzing the CSF proteome from different patients using unbiased proteomics approaches (e.g. Mass-spectrometry), novel CSF biomarkers candidates can be identified.
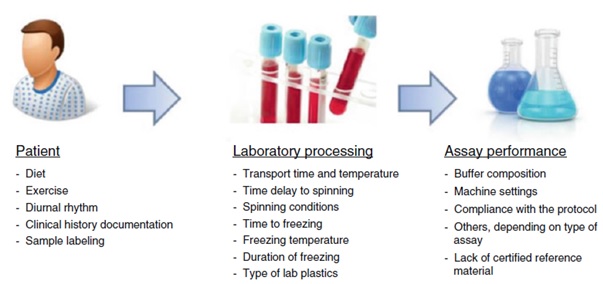
Despite the huge scientific efforts and investments employed in the development of novel CSF biomarkers for neurological diseases, the variability found across different studies have hampered their implementation in clinical practice. The intensive biomarker research performed in the last decade showed evidence that such variability is partly caused by the different ways of sample processing and biobanking followed by the different laboratories (figure 1), highlighting the importance of developing standard operating procedures (SOPs). One of the first international guidelines of CSF sample processing and biobanking were established by C. Teunissen and colleagues (2009) based on discussions that took place during the European network for MS biomarkers (bio-MS-EU) meetings. In the subsequent years, there was a growing interest in the effect of the pre-analytical confounding factors specifically on the classical AD, PD, and DLB CSF biomarkers (i.e. Tau, Amyloid-β, and α-Synclein). Therefore, the SOP and guidelines were frequently updated based on new evidence regarding the effect of the pre-analytical confounding factors on these CSF proteins. In our recently published book chapter, we discuss the established protocols with a special focus on the CSF proteome. Noteworthy, since experimental evidence regarding the influence of the pre-analytical confounding factors in most of the proteome is limited, we also aimed to investigate the stability of a large set of CSF proteins (>1000) after different delayed storage and freeze/thaw conditions using two novel large multiplex protein arrays (SOMAscan and Olink).
Over the course of ten years, the SOPs for CSF processing and biobanking have been implemented in in many biobanks world-wide, including ours. To achieve a decrease in variation across different studies, we need to continue our standardization efforts to keep increasing the quality of CSF samples stored in biobanks world-wide. Thus, more experiments and new updates to the current guidelines are expected in the future, especially with new emerging technologies that will facilitate the identification of novel CSF biomarkers. Identifying and controlling the influence of pre-analytical confounding factors on CSF protein analysis will eventually help in the development of successful CSF protein biomarkers for neurological diseases.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in