Gut Microbiota-Derived Siderophores as Key Modulators of PHD2 and HIF-1α Stability
Published in Biomedical Research and General & Internal Medicine
What are Prolyl Hydroxylase Domain Enzymes?
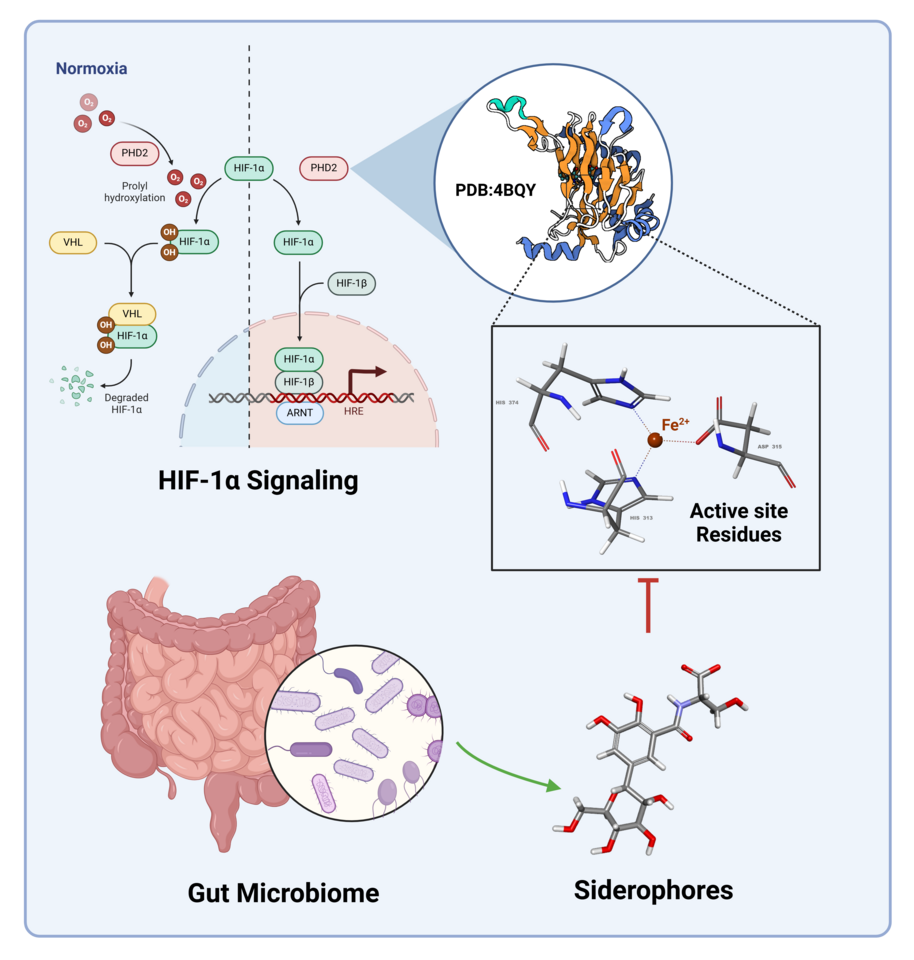
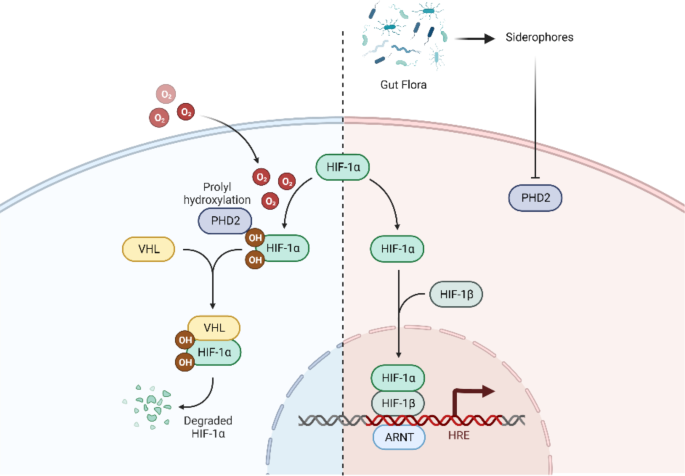
Prolyl Hydroxylase Domain (PHD) enzymes are the important oxygen-dependent ubiquitin ligases that target Hypoxia-Inducible Factor 1-alpha (HIF-1α) for degradation and thus control cellular adaptation to hypoxia. PHD enzymes convoke oxidation of HIF-1α, target for degradation under normoxic conditions. HIF-1α can thus be stabilized when PHDs, the oxygen-dependent degradation enzymes are inhibited thus triggering pathways that are vital when the cells need to adapt to hypoxic conditions in the body.
What are Gut Microbiota-Derived Siderophores?
Siderophores are small highly specific molecules used by the gut bacteria to sequester iron from its milieu. Apart from their involvement in microbial iron acquisition, these molecules display other surprising biochemical characteristics, including the binding to human enzymes. Specifically, some of the siderophores can chelate and interact with PHD2 reversibly providing a physiological control of HIF-1α stability.
Impact of Siderophore-PHD2 Interaction on HIF-1α Stability
Contemporary molecular docking and dynamics simulations have shown that natural siderophores including Salmochelin SX and Staphyloferrin A are potential PHD2 inhibitors among other siderophores. These molecules interact firmly with ASP254, TYR310, ASP315, and ARG322, which are necessary for its active site. Based on the binding pose metadynamics analysis it was observed that although Salmochelin SX has the highest binding affinity (-9.527 kcal/mol).
Why Is This Important?
The influence of gut microbiota derived siderophores together with Prolyl Hydroxylase Domain 2 (PHD2) is a major step forward in deciphering hypoxia signaling networks molecularly. This is particularly important when focusing on ischemic strokes where oxygen becomes scarce, and tissues of the brain are compromised heavily. While tightening feedback-controlled degradation of HIF-1α by inactivation of PHD2 can activate protective adaptive signaling necessary for tissue survival, Angiogenesis and Metabolic Reprogramming in ischemic conditions.
Here, the investigators describe a physiological process thru which the gut microbiome may impact systemic responses to hypoxia, further elucidating the Gut-PHD2-HIF-1α pathway. An understanding of this relationship is essential to asking questions about the nature of the gut-brain connection and its role in stroke, a condition where hypoxia plays a major role.
Future Directions
The identification of siderophores produced by gut microbiota as PHD2 inhibitors indicates rich potential for controlling ischemic stroke through the HIF-1α pathway. Ischemic stroke which results from the obstruction of blood supply to the brain results in grave hypoxic and ischemic damage. Stabilization of HIF-1α is also an attractive target for therapy because it activates hypoxic target genes involved in cell survival, angiogenesis and metabolic adaptations to reduced oxygen availability. These adaptive reactions are done by means of preventing degradation of HIF-1α by binding of its PHD2 catalytic domain by siderophores. Those points offer clues for preventing neuronal damage and reducing the size and progression of infarction and tissue repair in stroke patients.
Subsequent work should examine the therapeutic properties of siderophores and their derivatives in experimental ischemic stroke: applicability, effectiveness, neuroprotection, and recovery. More knowledge regarding the structural features of siderophores that need to be in place to strongly inhibit PHD2 will benefit the generation of selective treatments. Taken collectively, these results propose that targeting Gut-PHD2-HIF-1α axis might offer a novel strategy for treating ischemic stroke and other hypoxia-associated diseases.
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Women’s Health
Publishing Model: Open Access
Deadline: Feb 28, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in