Happy Killers in Hostile Tumors: Engineering T Cells with a Built-In Survival Toolkit
Published in Cancer and Immunology
The immune system protects us from many threats, including our own cells when they grow out of control. However, when cancer develops, immune control is lost and can even be reversed to the point that the immune system may support tumor growth.
To re-educate the patient’s immune system to recognize and attack cancer, cancer immunotherapies are now integrated into most treatment schedules. One of the most recent strategies is T-cell engineering. In this approach, scientists extract T cells from the patient’s blood and train them in the laboratory to detect and respond to tumor cells by equipping them with specialized “antennas,” called receptors. These receptors come in different shapes and sizes, but the concept is the same: once the receptor binds its target on the tumor cell, it delivers a signal, known as T-cell activation signal 1, that prompts the T cell to act and kill the tumor cell.
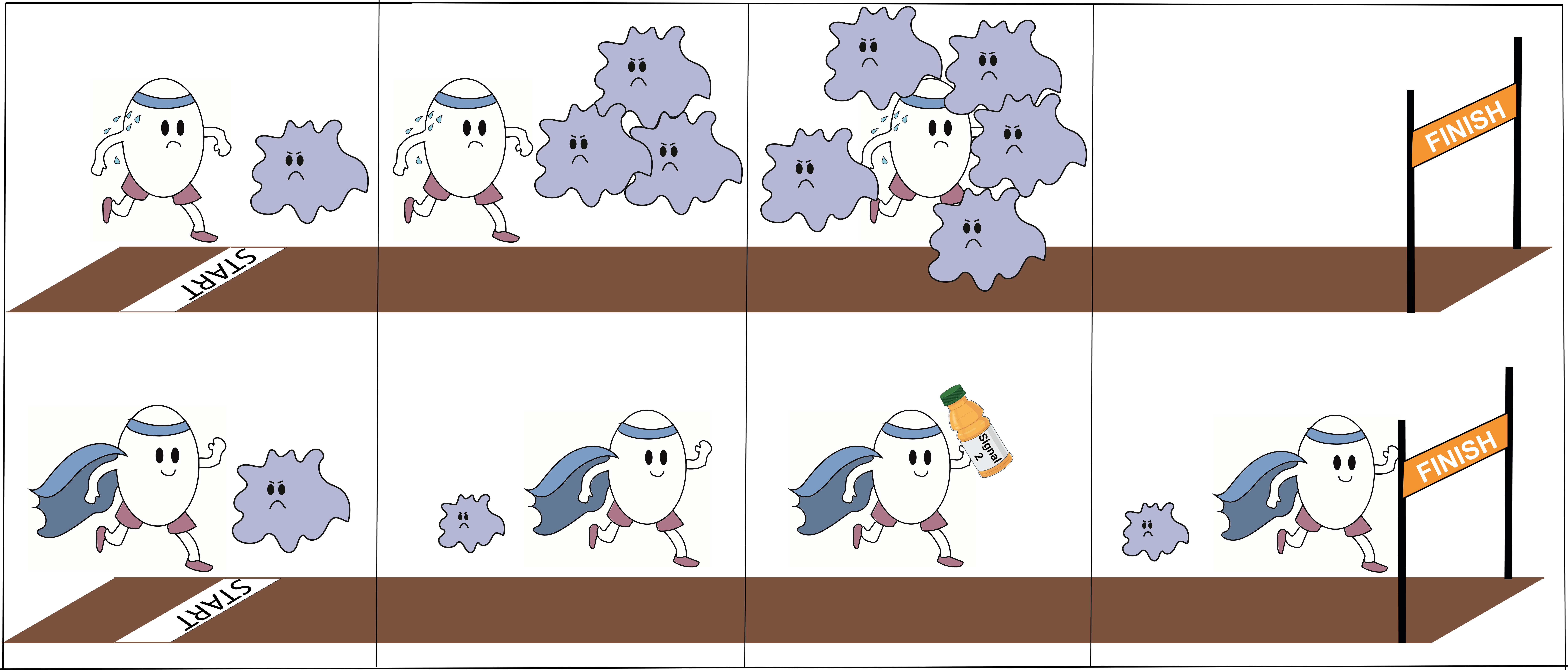
Engineered T cells often behave like sprinters: once their receptor is triggered by a target molecule on the tumor surface, they rapidly begin attacking and killing tumor cells. However, they do not run on a smooth track but in a crowded marathon, the tumor microenvironment, which drains their energy and leads to exhaustion and death. This is one of the reasons why tumors often return even after initially successful immunotherapy.
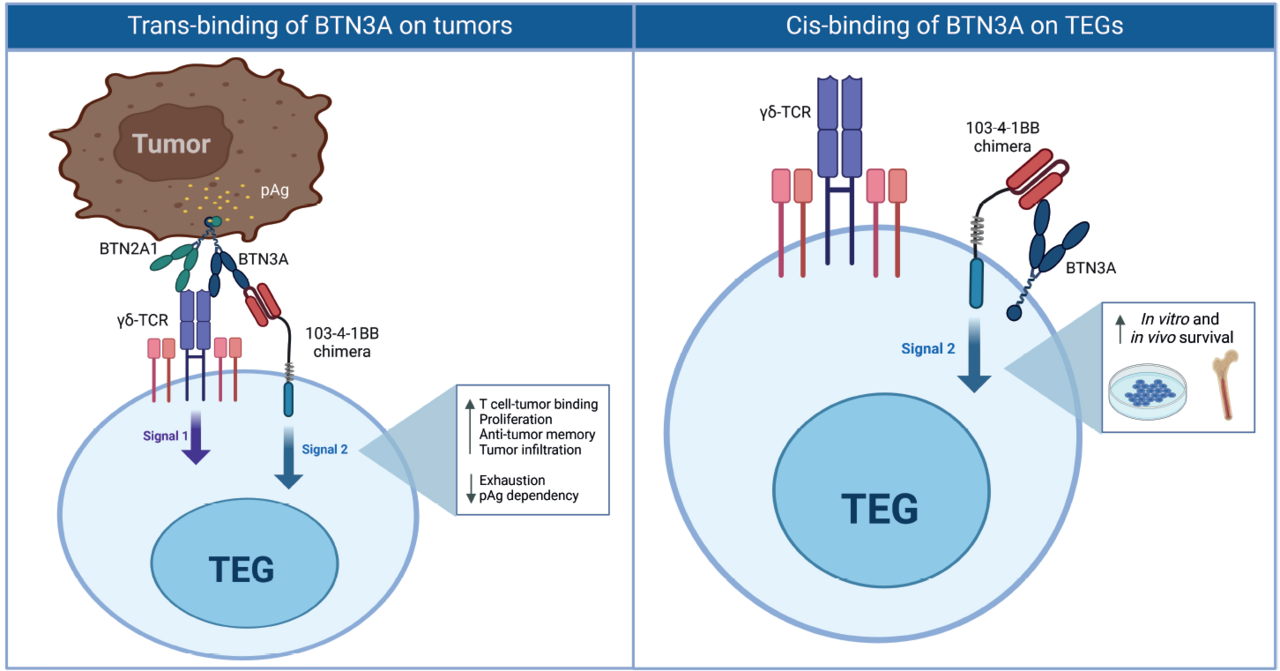
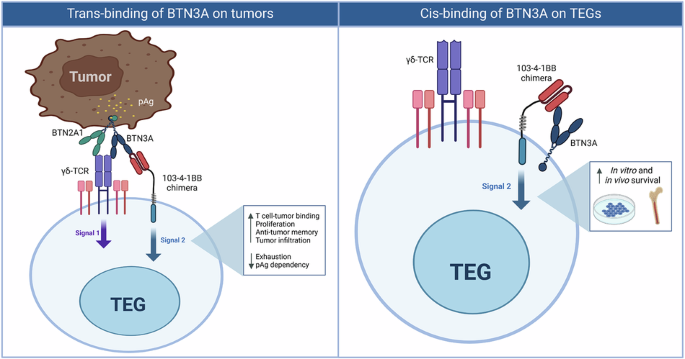
We therefore wondered whether we could engineer T cells with the stamina of a marathon runner while retaining the power of a sprinter. To achieve this, we equipped T cells with an additional receptor that provides an activation boost known as T-cell activation signal 2, helping them stay energized. We designed this receptor to recognize and bind a specific molecule, BTN3A, highly expressed in the crowded tumor environment. When this receptor binds BTN3A, it activates the signal-2 power boost, fueling the T cell to run the long tumor-fighting marathon. This strategy enabled engineered T cells to attack cancer cells more effectively, penetrate deeper into tumors, and, crucially, avoid exhaustion. In our mouse models, these engineered T cells were able to completely eliminate tumors.
The biggest surprise came when we found that the engineered T cells not only cleared the tumors but were still alive more than 100 days later, long after the tumors had disappeared. Their additional receptor appeared to keep them unusually “happy” and energized.
This unexpected persistence made us curious about the underlying mechanism. We discovered that BTN3A is present not only on tumor cells but also at low levels on the engineered T cells themselves. This self-binding kept the engineered T cells energized even in the absence of tumors. As a result, they remained ready to run the tumor-fighting marathon again and again. Indeed, when mice were re-challenged with tumors, the surviving engineered T cells immediately sprang into action and efficiently eliminated them. With this approach, the engineered T cells carry their own self-sustaining survival toolkit, allowing them to remain active, resilient, and ready to fight.
Looking ahead, our strategy has the potential to overcome major hurdles that currently limit T-cell therapies, especially against solid tumors. Its adaptability to other cellular therapies further opens new opportunities in cancer immunotherapy. Ultimately, our study highlights a key principle: in the tumor-fighting marathon, resilience matters more than speed.
Follow the Topic
-
Cellular & Molecular Immunology

A monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, covering both basic immunology research and clinical applications.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in