Helminths, microbiomes, and antimicrobial resistance: A real-world scenario.
Published in Microbiology

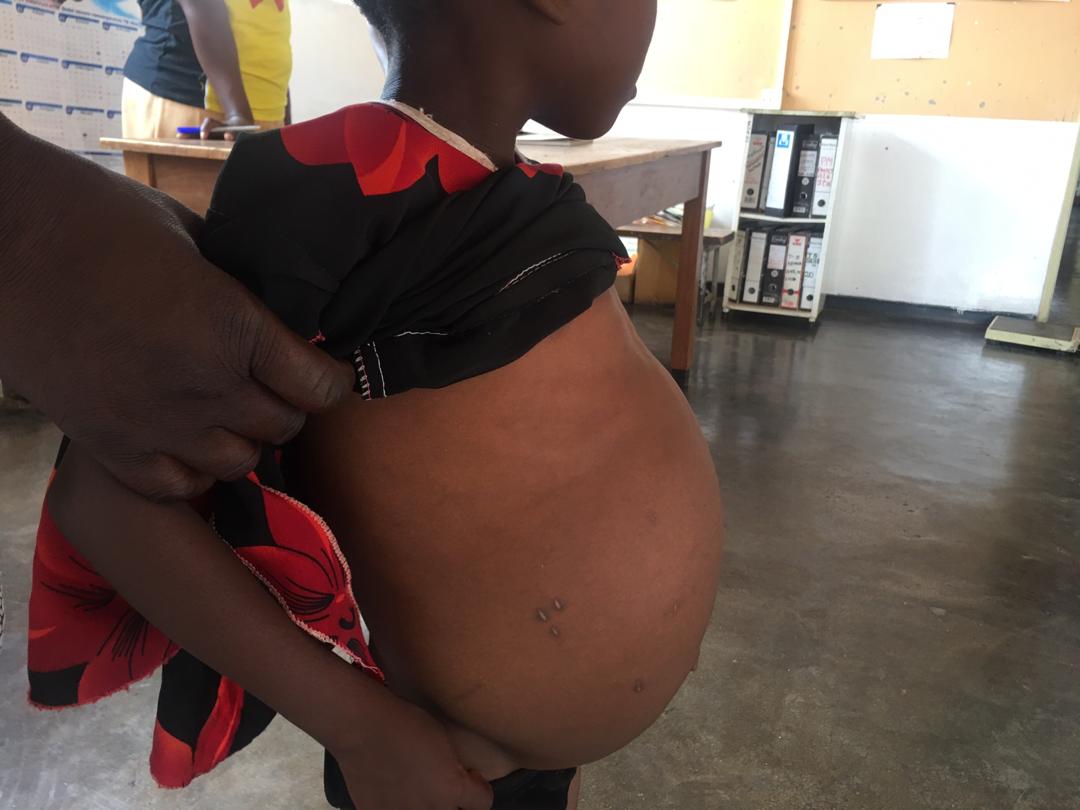
Schistosomiasis, also known as Bilharzia, is a parasitic disease, mainly poverty-related, with more than 90% of affected populations living in sub-Saharan Africa. It is one of the Neglected Tropical Diseases (NTDs), acquired when people come into contact with the infective forms (cercariae) of blood flukes known as schistosomes, often found in fresh water. A large proportion of children are infected, with consequences including malnutrition, stunted growth, anaemia, poor performance in school, and organ involvement, which in serious cases, can lead to fatalities. The World Health Organisation (WHO) has just published the new 2021–2030 NTD roadmap, setting global targets to prevent, control and eliminate NTDs; the notable targets for schistosomiasis indicate that critical action is required. In support, the Uniting to Combat NTDs coalition has declared 2020 as the NTD year, and launched the “End the Neglect” campaign to help with the cost of implementing the WHO’s NTD roadmap. Scientific research continues to explore our understanding into the health consequences of schistosomiasis, and studies to determine more of the systemic impacts of infection, and how this relates to disease and poor health are essential.
The human gut comprises a diverse ecosystem of microbes (microbiota) – bacteria, viruses, fungi and other eukaryotes. We rely on this mutual relationship with microbes to help absorb essential nutrients from food, as a first line of protection from pathogens, and in shaping the immune system through the relay of important chemical signals. This ecosystem is also known to be a major reservoir for antimicrobial resistance genes (the resistome); a potential threat to treatment of many major infections. However, when humans become infected with a pathogen, like in schistosomiasis, this relationship may be disturbed, and this may not work in our favour. Although still expanding, the field of metagenomics research has allowed us insights into the microbiome (the microbiota, their genetic material, and environmental interactions), and how we can apply this to further understand health and disease in diverse populations.
In infants and young children, the microbiome evolves until about age 3 to 5 years, and the infections that children may be exposed to, are key to shaping that microbiome. Consider, for example, a case where a preschool child (less than 5 years old) living in a schistosome-endemic area, gets infected with Schistosoma haematobium, the causative agent of the urogenital form of schistosomiasis. In addition to the known consequences, schistosome infection is likely to threaten the stability of the microbiome, and its associated role in the health and development of that child. In a recent study published in Communications Biology (read full paper here), we take a closer look at the systemic impacts of infection with Schistosoma haematobium on the microbiome and resistome of preschool children (<5 years old) in Zimbabwe. For the first time, we identified significant differences in the gut microbiome but not the resistome (despite evidence of over 260 antimicrobial resistance (AMR) genes), of S. haematobium-infected and uninfected children, while controlling for epidemiological and clinical factors. Overall, our analysis suggests microbiome disturbances as an added consequence of S. haematobium infection, relevant to disease progression and poor health.

Why is this important?
Given that the microbiome in infants and young children is still developing, such changes could have significant impacts on the establishment of the gut microbiome and its role in overall future health. This adds to our understanding into the molecular processes underlying schistosomiasis in preschool children, i.e. 5 years old and below, and to the calls for early inclusion and treatment of this age group in schistosome control programmes. Our findings will have a major impact on helminth infection control in endemic areas by first, helping to provide new strategies to reduce schistosome-associated pathology, and secondly, providing an understanding into the role of nutraceuticals in influencing health through the microbiome, for selected helminth infections.
Although we found that the changes in the microbiome was not associated with the resistome, it is in line with suggestions that many inherent population anthropogenetic (of or relating to their origin and development) and environmental factors greatly drive the resistome, and all of these factors have major implications on formulating AMR policy. Zimbabwe recently launched a One Health Antimicrobial Resistance National Action Plan. Hence in keeping with calls from the WHO and the United Nations on strategic policies on antibiotic use, scientific information comparable to the data provided by this study will help formulate policy and guide revisions in antibiotic use in the country, and for the rest of sub-Saharan Africa.
In addition, this age group is understudied, and thus our study contributes to the repository of information on microbiome and AMR studies, by providing a "novel" metagenomics dataset of preschool children from a developing country (details of data availability can be found in the published article).
As our knowledge in the field of the microbiome expands, so does our understanding of the complex relationship it holds with the immune system and its role in health and disease. Unlocking such interactions could be key to future solutions in an evolving healthcare system.
Derick N. M. Osakunor Follow on twitter @dosakunor

The work was conducted by the Parasite Immuno-epidemiology Group and resident bioinformatician, Dr. Alasdair Ivens at the University of Edinburgh, and collaborators at the University of Zimbabwe, and at the Denmark Technical University.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in