Hidden Aspects of Cortical Dysfunction in Parkinson's Disease Revealed
Published in Neuroscience

Symptoms of Parkinson's disease (PD) are often explained by the loss of dopamine, which disrupts brain circuits controlling movement, including the cerebral cortex— the brain's outer structure [1, 2]. However, emerging scientific evidence suggests that functional impairments in the cerebral cortex can be caused directly by the buildup of an abnormally aggregated protein called α-synuclein, not just by dopamine loss. This discovery could lead to new ways of treating PD.
What We Wanted to Learn
We wanted to understand how α-synuclein, which clumps together in PD, affects brain cells in the cortex. Previous studies showed that dopamine loss in the subcortical regions triggers changes in subtypes of cortical neurons and their connections [3, 4]. Yet, systemic investigations of the effects of abnormally aggregated α-synuclein on cortical circuits remain lacking. This is a critical gap in our knowledge to understand the biology underlying cognitive impairment in PD and other synucleinopathies.
How We Studied It
We used a mouse model of PD, where we injected pathological α-synuclein seeds into a specific brain region called the striatum. This is a widely used animal model of α-synuclein pathology and induces robust Lewy-like pathology in broad brain regions in a time-dependent manner, including the cerebral cortex [5, 6]. Using this model system, we employed several sophisticated research approaches to rigorously study the morphology, electrophysiology, and synaptic properties of cortical neurons. We reported striking differences in cellular excitability by comparing cells that are vulnerable to versus those that are more resilient to α-synuclein pathology.
Key Findings (Figure. 1)
- Subtypes of Cortical Neurons Are More Affected: α-Synuclein aggregates impact certain cells (intratelencephalic neurons, or ITNs) earlier and more severely than others (corticospinal neurons, or CSNs).
- Cellular hyperexcitability: The cortical neurons bearing α-Synuclein aggregates become hyperexcitable. These cells are intrinsically more excitable because they discharge more action potentials when most synaptic connections have been blocked.
- Structural Damage: The α-Synuclein aggregates-bearing cells show morphological changes, including fewer branches and connections (spines) and smaller cell bodies.
- Ionic mechanisms: We demonstrate that the hyperexcitability of cortical neurons is largely caused by the downregulated function of the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels.
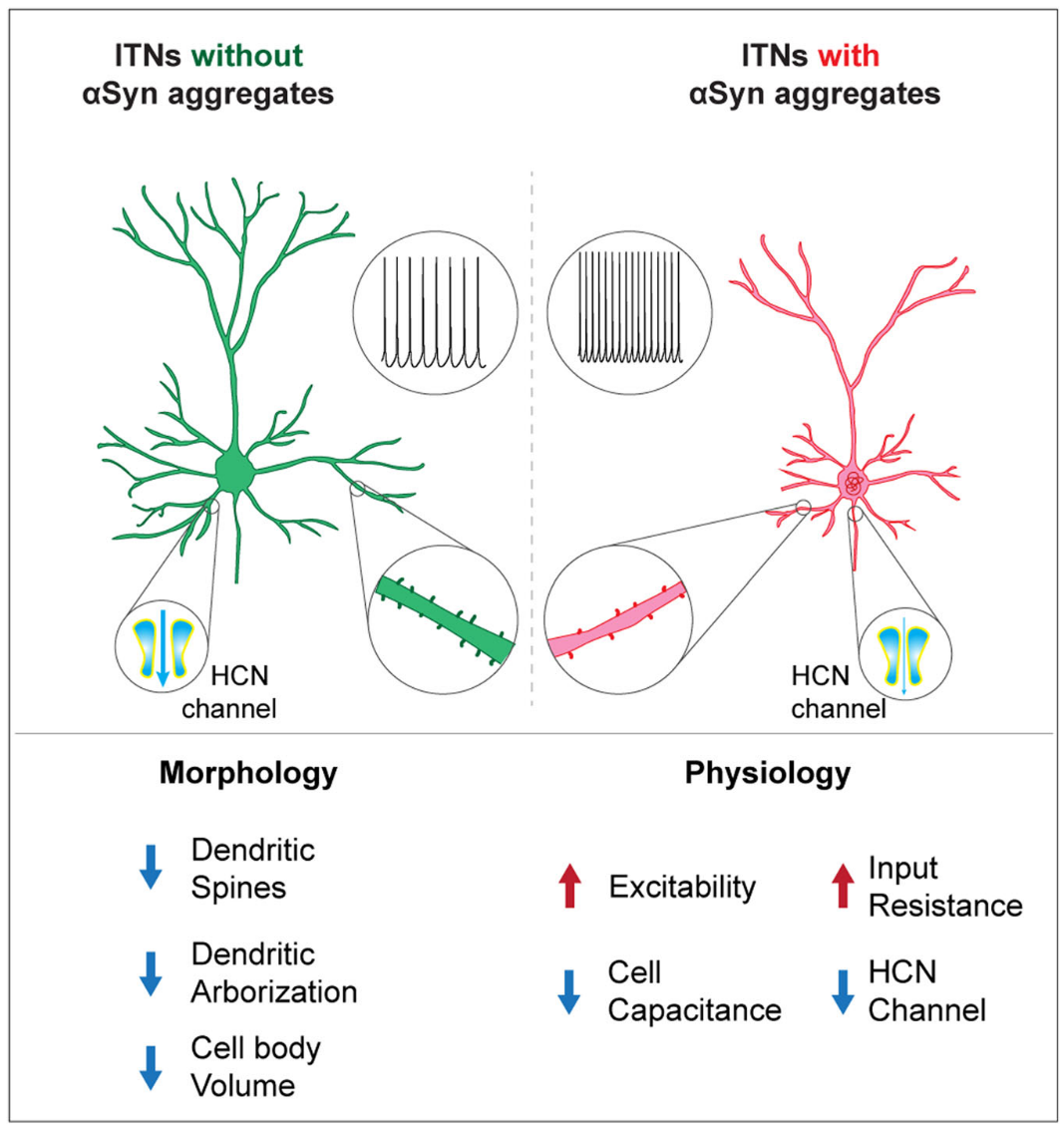
Figure. 1 | A graph summarizing the morpho-electrophysiological changes of cortical neurons associated with αSyn pathology. The formation of cytoplasmic αSyn aggregates changes the morphological and physiological properties of cortical pyramidal neurons via cell-autonomous mechanisms, involving a downregulated HCN channel function.
Surprising Results
Our earlier work reported that loss of dopamine selectively affects pyramidal tract neurons in the motor cortex and their excitatory inputs from the thalamus. This work, and others, show that the intratelencephalic neurons in the motor cortex and their excitatory inputs from other cortical regions are vulnerable to α-Synuclein pathology. Together, these exciting data show that α-synuclein pathology and dopamine loss damage the brain function in different manners.
Challenges We Faced
One difficulty was identifying which cells had α-synuclein clumps during our experiments. To solve this, we had to label and post hoc examine each individual cell under the microscope to ensure the formation of protein aggregates, which was time-consuming. This is a nice example demonstrating our effort in conducting rigorous scientific research.
Why This Matters
It is well-acknowledged that PD affects the cerebral cortex. The traditional model of the cortico-basal ganglia network can explain some aspects of cortical dysfunction following the degeneration of midbrain dopamine neurons. The present work reveals hidden aspects of cortical dysfunction associated with Lewy-like pathology. Such an in-depth understanding of cortical network dysfunction is needed to design more effective treatments for PD patients.
References
- McGregor, M.M. and A.B. Nelson, Circuit Mechanisms of Parkinson's Disease. Neuron, 2019. 101(6): p. 1042-1056.
- Galvan, A. and T. Wichmann, Pathophysiology of parkinsonism. Clin Neurophysiol, 2008. 119(7): p. 1459-74.
- Chen, L., et al., Cell Type-Specific Decrease of the Intrinsic Excitability of Motor Cortical Pyramidal Neurons in Parkinsonism. J Neurosci, 2021. 41(25): p. 5553-5565.
- Chen, L., et al., Reduced thalamic excitation to motor cortical pyramidal tract neurons in parkinsonism. Sci Adv, 2023. 9(34): p. eadg3038.
- Luk, K.C., et al., Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science, 2012. 338(6109): p. 949-53.
- Chen, L., et al., Synaptic location is a determinant of the detrimental effects of alpha-synuclein pathology to glutamatergic transmission in the basolateral amygdala. Elife, 2022. 11.
Follow the Topic
-
npj Parkinson's Disease

This journal publishes original basic science, translational and clinical research related to Parkinson's disease, including anatomy, etiology, genetics, cellular and molecular physiology, neurophysiology, epidemiology and therapeutic development and treatments.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Cognition - preclinical models, and preclinical unmet need
Publishing Model: Open Access
Deadline: Jul 27, 2026
Environmental risk factors for Parkinson’s disease
Publishing Model: Open Access
Deadline: May 13, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in