Hierarchical self-assembly and emergent function of densely glycosylated peptide nanofibers
Published in Bioengineering & Biotechnology

Carbohydrates are the most abundant organic molecules on earth.
In our daily lives, we encounter a vast array of natural entities assembled from carbohydrates. Cellulose, a polymer of glucose, is the primary structural component of the plant cell wall, while chitin is a key component of the exoskeletons of insects and crustaceans. Likewise, nearly half of all mammalian proteins are modified by carbohydrates (i.e., glycosylated), which can guide their organization into complex functional superstructures, as seen for mucus. Yet, employing carbohydrates to assemble synthetic biomaterials has lagged far behind the use of other biomolecules, such as amino acids, lipids, and nucleotides, from which a diverse array of synthetic architectures can be realized.1 Our team at the University of Florida reports in Communications Chemistry (https://rdcu.be/bAv0N) that glycosylation can direct the self-assembly of peptide nanofibers into highly ordered networks with emergent functional properties (Figure 1).
This project began a few years ago when a conference attendee asked me if glycosylated peptide nanofibers developed by our team could be crosslinked by proteins with multiple carbohydrate-binding sites. Early experiments conducted by Dr. Antonietta Restuccia suggested yes, but a series of control experiments resulted in an unexpected observation -- glycosylated peptide nanofibers formed aligned bundles in the presence of every protein tested (as seen in the banner image above), regardless of whether the protein had carbohydrate-binding sites or not. Intrigued by this, we enlisted experts in structural biology (Kelley & Kurian) and soft matter (O’Bryan & Angelini) to help deconstruct this process.
Nanofibers assembled from peptides often aggregate into random entangled networks in water. We observed that attaching a carbohydrate onto the peptide can prevent this aggregation, likely by enhancing water-nanofiber interactions. Moreover, the carbohydrate can mediate specific interactions between nanofibers that direct their lateral association and alignment. These interactions depend on the type of sugar, as well as its density, on the nanofiber. For example, nanofibers modified with N-acetyllactosamine align to form bundles, whereas those modified with the variant N’,N-diacetyllactosamine remain dispersed.
Networks of aligned glycosylated peptide nanofibers also demonstrate new functional properties that are not shared by their non-glycosylated counterparts. For example, nanofibers modified with N-acetylglucosamine provide biomaterials that are resistant to fouling by bacteria and mammalian cells, yet selectively capture carbohydrate-binding proteins, similar to mucus and other natural glycosylated superstructures.
Analogous to the influence of glycosylation on protein form and function, this report demonstrates that carbohydrate modifications can also endow new properties to synthetic peptide assemblies. Our observations add to a growing body of work highlighting the potential of sugars as components in synthetic supramolecular biomaterials.2,3 Moving forward, we envision that modifying peptides with the diverse carbohydrate chemistries found throughout nature will lead to supramolecular biomaterials with a broad range of emergent properties that are useful for various medical and biotechnological applications.
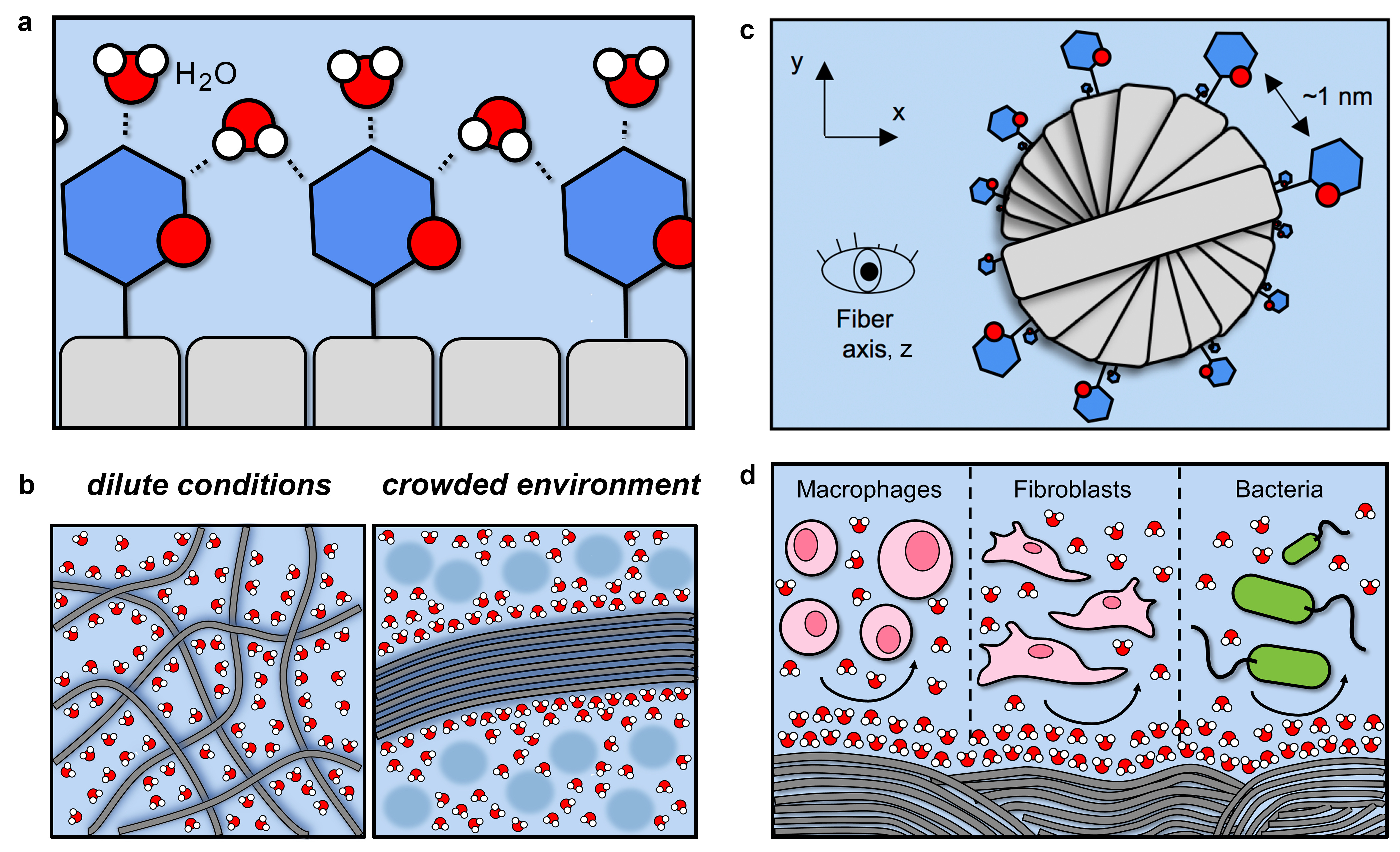
Figure 1. Overview of hierarchical self-assembly and emergent function of densely glycosylated peptide nanofibers. (a-b) Carbohydrate-water interactions prevent nanofiber aggregation under dilute conditions, while excluded volume effects drive nanofiber bundling in crowded environments. (c) Carbohydrates radially projected at regular intervals provide inter-fiber contact points in all directions that facilitate lateral association without geometric constraint. (d) Glycans on the surface of nanofibrillar biomaterials prevent non-specific mammalian cell and bacteria adhesion.
References
- Webber, M.J., Appel, E.A., Meijer, E.W., Langer, R. Supramolecular Biomaterials. Nature Materials (2016) 15, 13-26. DOI: doi.org/10.1038/nmat4474
- Yu, Y., Gim, S., Kim, D., Arnon, Z.A., Gazit, E., Seeberger, P.H., Delbianco. Oligosaccharides Self-Assemble and Show Intrinsic Optical Properties. Journal of the American Chemical Society (2019) 141, 4833-4838 DOI: doi.org/10.1021/jacs.8b11882
- Liu, R. Hudalla, G.A. Using Self-Assembling Peptides to Integrate Biomolecules into Functional Supramolecular Biomaterials. Molecules (2019) 24, 1450 DOI: doi.org/10.3390/molecules24081450
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Advances in Asymmetric Catalysis for Organic Chemistry
Publishing Model: Open Access
Deadline: Mar 31, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in