Highly potent and broadly neutralizing anti-CD4 trimeric nanobodies inhibit HIV-1 infection by inducing CD4 conformational alteration
Published in Microbiology
The Road to Discovery:
In our ongoing quest to find more effective therapies against HIV-1, we focused on the potential of nanobodies—small, single-domain antibodies derived from camelids like alpacas. Despite advances in antiretroviral therapy (ART), challenges such as lifelong medication, side effects, and the development of drug-resistant viral strains persist, making the search for novel therapeutic approaches imperative. CD4, the primary receptor for HIV-1 entry into host cells, presents a compelling target, yet its potential has not been fully realized.
Our breakthrough came with the identification of Nb457, which demonstrated exceptional potency and a broad spectrum of activity against HIV-1. Nb457’s ability to induce a conformational change in CD4, thereby blocking viral entry without impairing CD4+ T cell function, set it apart from existing therapies, including Ibalizumab, the only approved CD4-specific antibody.
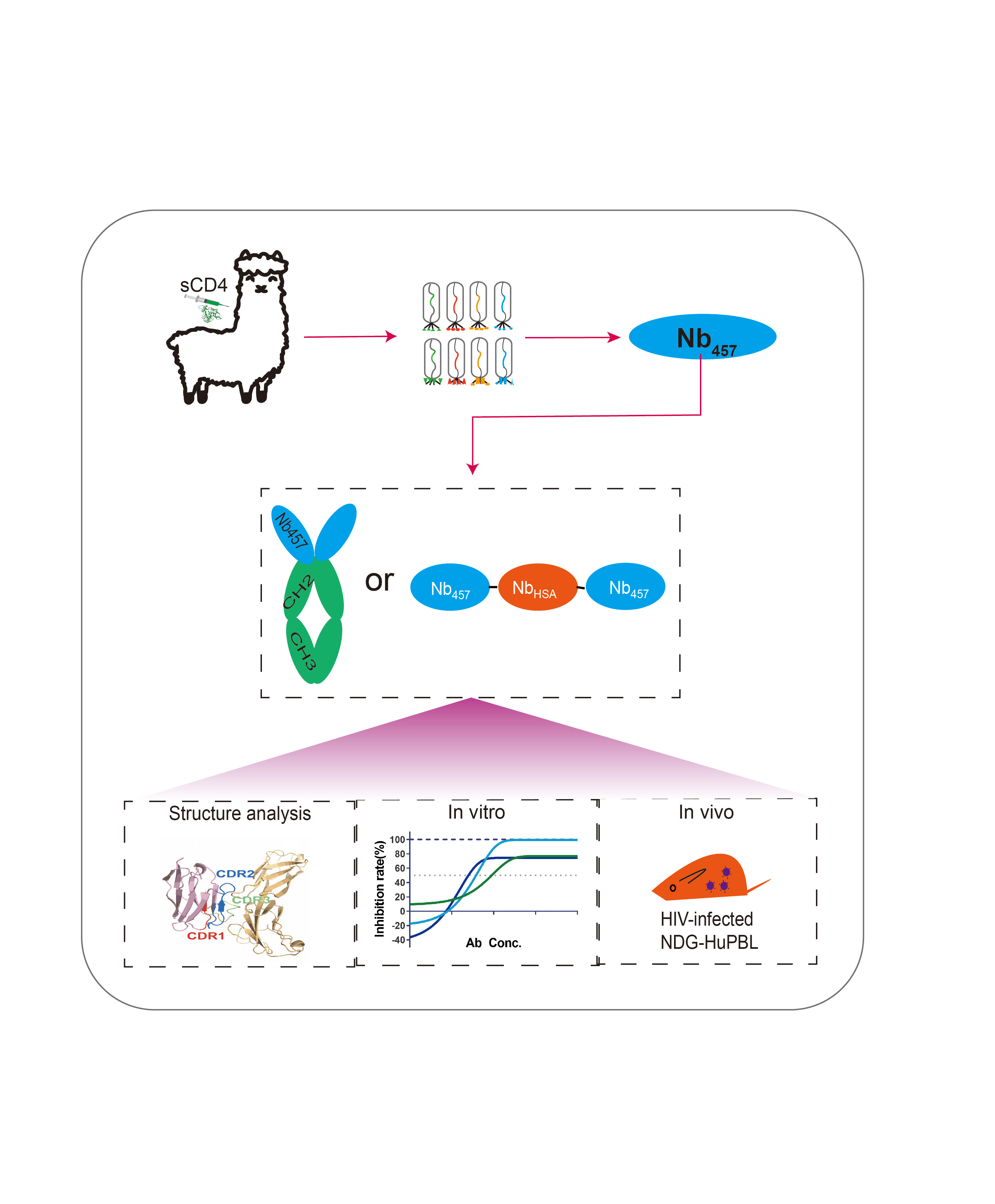
Engineering for Potency:
Building on this discovery, we engineered a trimeric form of Nb457 (Nb457-NbHSA-Nb457) that demonstrated complete inhibition of live HIV-1 in our studies. This trimeric nanobody outperformed both Ibalizumab and the parental Nb457, achieving an impressive 100% inhibition rate against various HIV-1 strains. The structural analysis revealed that Nb457 binding caused a conformational change in CD4, preventing the virus from binding and entering host cells.
In Vivo Efficacy:
The efficacy of Nb457 was not just confined to in vitro studies. In humanized mouse models, Nb457 exhibited significant therapeutic effects against HIV-1 infection. Particularly, the trimeric Nb457 showed superior efficacy, with some treated mice achieving nearly undetectable viral loads. These findings underscore the potential of Nb457 as a potent and safe therapeutic option for HIV-1, capable of targeting a wide range of viral strains, including those resistant to current therapies.
Looking Ahead:
Our study highlights the immense potential of nanobodies, particularly Nb457, in the development of new HIV-1 therapies. The small size, high thermal stability, and specificity of nanobodies make them ideal candidates for various biomedical applications, including gene therapy and antibody-drug conjugates (ADCs). Moreover, the ability to produce nanobodies in more cost-effective systems like yeast or bacteria could make these therapies more accessible to a broader population, addressing one of the critical barriers to the widespread adoption of antibody-based treatments.
Conclusion:
The identification and development of Nb457 as an ultra-potent anti-CD4 nanobody represent a significant advancement in HIV-1 therapeutics. With its exceptional potency, broad-spectrum activity, and favorable safety profile, Nb457, particularly in its trimeric form, stands as a promising candidate for further research and clinical development. As we continue to explore the potential of this nanobody, we are optimistic that it could revolutionize the treatment landscape for HIV-1, bringing us one step closer to a functional cure for this persistent global health challenge.
Reference link: https://www.nature.com/articles/s41467-024-51414-6
Reference
- Emu, B. et al. Phase 3 Study of Ibalizumab for Multidrug-Resistant HIV-1. Engl. J. Med. 379, 645-654, doi:10.1056/NEJMoa1711460 (2018).
- Wu, X. et al. Tandem bispecific neutralizing antibody eliminates HIV-1 infection in humanized mice. J Clin Invest 128, 2239-2251, doi:10.1172/JCI96764 (2018).
- Huang, Y. X. et al. Engineered Bispecific Antibodies with Exquisite HIV-1-Neutralizing Activity. Cell 165, 1621-1631, doi:10.1016/j.cell.2016.05.024 (2016).
- Pace, C. S. et al. Bispecific antibodies directed to CD4 domain 2 and HIV envelope exhibit exceptional breadth and picomolar potency against HIV-1. P Natl Acad Sci USA 110, 13540-13545 (2013).
- Walsh, S. R. & Seaman, M. S. Broadly Neutralizing Antibodies for HIV-1 Prevention. Frontiers in immunology 12, doi:ARTN 71212210.3389/fimmu.2021.712122 (2021).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in