How dissecting pathogen virulence can help predict the outcome of infection
Published in Microbiology

Upon infection, a host will try to reduce pathogen numbers by producing an immune response towards them. However, depending on various factors, such as host-pathogen genotypes (e.g., coevolutionary history) or host energetic reserves, it might not be able to clear them (Figure 1). In short, there are three different infection outcomes: a) the host limits pathogen growth and clears the infection; b) pathogen growth impairs host homeostasis, likely leading to its death; or c) the host limits pathogen numbers but does not clear them, perhaps because it is not able to (e.g., pathogen persister cells are able to evade immune surveillance) or because the host does not energetically compensate in the long-term. Immunity is costly to the host, requiring re-allocation of resources from other functions to immune defences, without an always clear positive outcome [1]. This has become particularly apparent in the last decade, when the focus in studies of animal hosts has turned to infection tolerance, rather than only considering resistance [2].
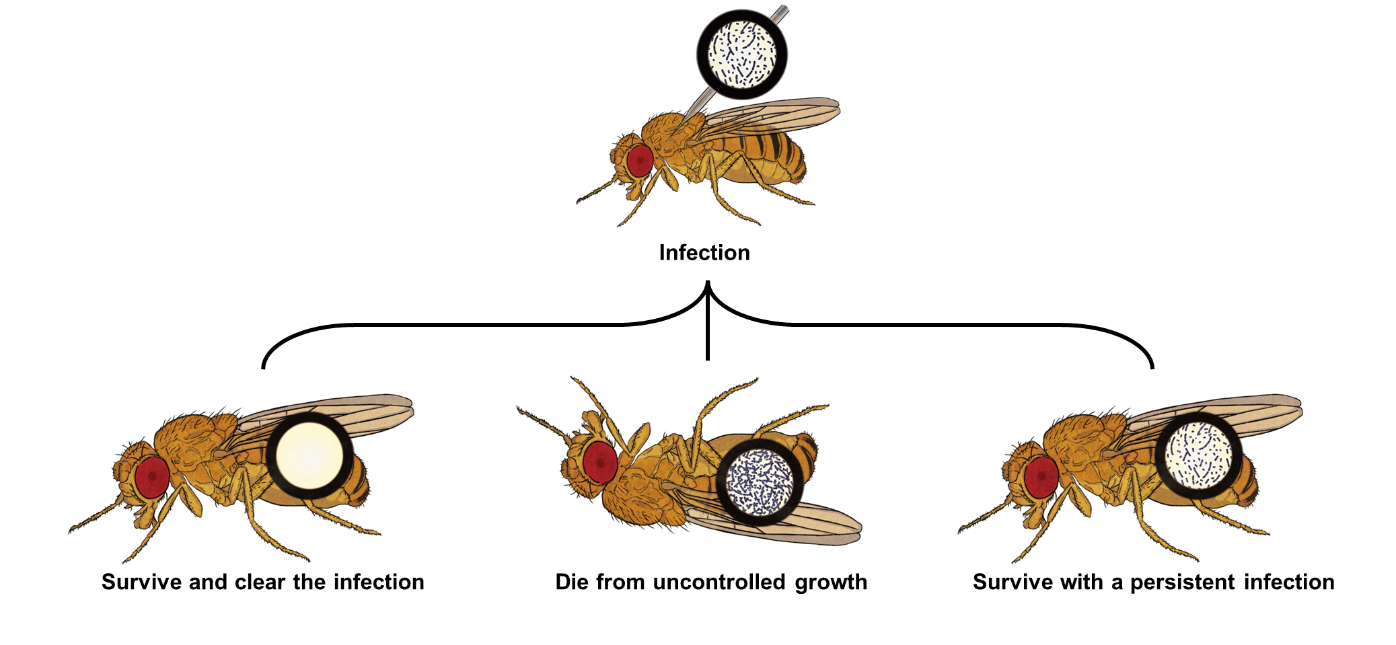
Figure 1. Infection outcomes. Previous literature on D. melanogaster bacterial infections has acknowledged two main infection outcomes [7], death by pathogen over-proliferation or survival with a persistent infection. In our study, we show that infection clearance is an equally important infection outcome that was observed across bacterial species that vary in virulence.
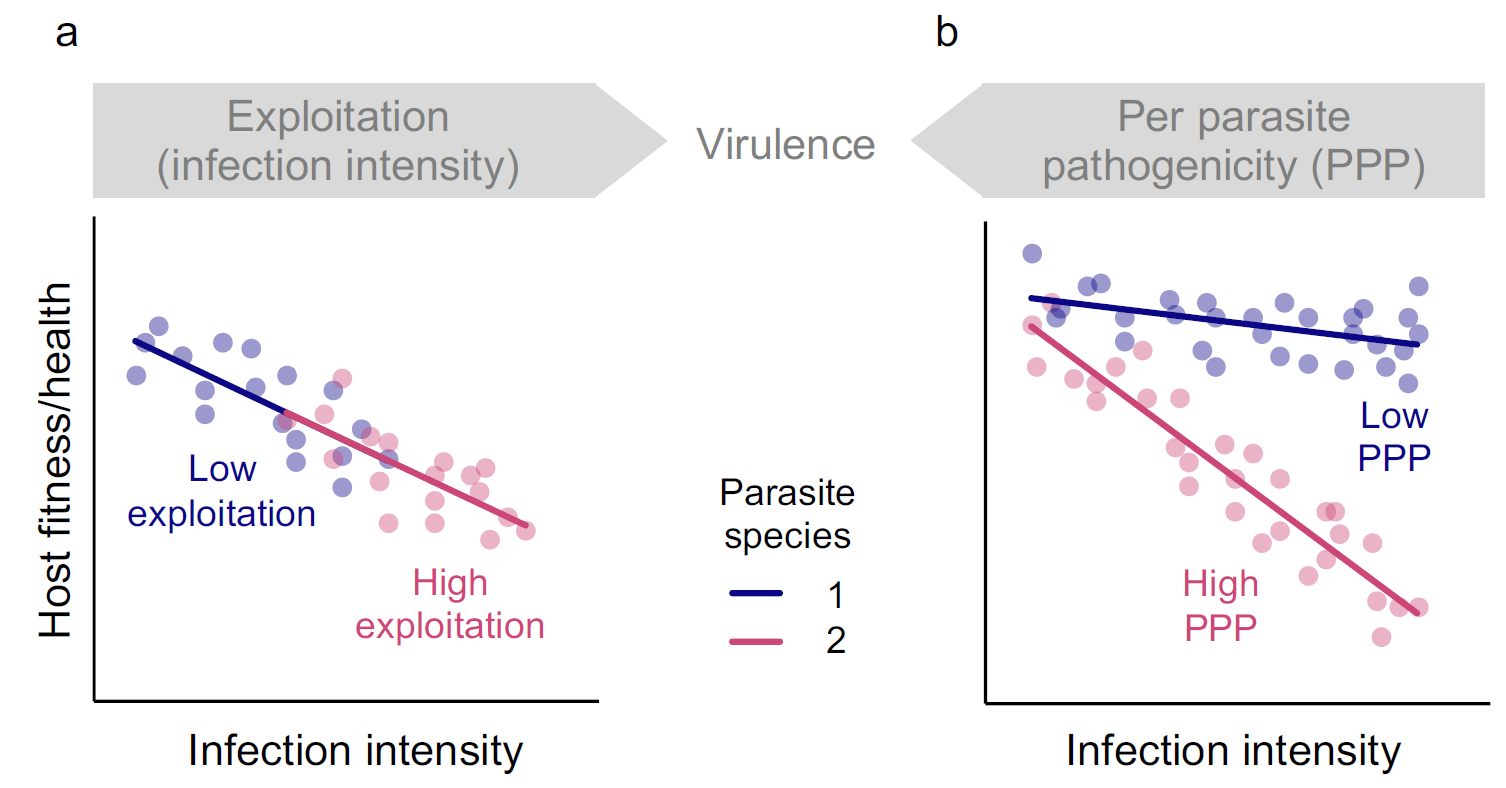
Figure 2. Decomposing virulence. Both exploitation and per parasite pathogenicity (PPP) can harm the host and thereby contribute towards virulence. Exploitation describes the infection intensity, or bacterial load, inside the host. PPP describes the damage per parasite that an infection does to the host independently from the pathogen load (e.g., toxins). Here there is variation among pathogen species in exploitation and PPP, as illustrated by hypothetical relationships between host fitness and infection intensity for two species of parasite infecting the same host genetic background. a. The parasite species have the same PPP but differ in exploitation. Parasite 1 has lower exploitation compared with parasite 2, because it causes a lower infection intensity. b. In contrast with a, the parasite species have the same average exploitation but species 1 has lower PPP because its reaction norm has a shallower slope. This means that compared with species 2, species 1 causes less damage to the host with increasing parasite load. Figure modified from Råberg & Stjernman [4].
A classically studied property of a pathogen is its virulence. Traditionally, immunology literature has characterised virulence as a “genetic term for the parasite-induced reduction in host fitness” [3]. Nevertheless, it takes two to tango and therefore, virulence results from both pathogen and host properties; for instance, a pathogen might develop differently within two hosts with different genotypes, depending on how well it is adapted to complete its life cycle inside each host. In light of this, Råberg and Stjernman (2012) described virulence as a balance between host and parasite components, that will influence the infection outcome [4]. Hosts encompass two conceptually different defence strategies to the pathogen, resistance, which includes immune responses aimed to decrease pathogen numbers (e.g., antimicrobial peptide production), and tolerance, which reduces the fitness or health cost of sustaining and fighting an infection [5]. On the parasite side, exploitation (Figure 2a) is characterised by pathogen numbers and how many host resources are sequestrated by its growth, and per parasite pathogenicity (PPP) (Figure 2b), meaning the amount of harm a pathogen is able to cause independently of its numbers. This framework has been applied in the context of HIV infections [6], where it was shown that the viral genotype affects virulence and infection outcome mainly through PPP modulation rather than exploitation. Prior literature mostly focused on studying different populations/genotypes within the same species. In our study, we expanded the use of this framework by using four opportunistic bacterial species with different degrees of virulence (Figure 3) and tested if the bacterial components of virulence, i.e., exploitation and PPP, help us to understand and predict the outcome of these dissimilar infections in Drosophila melanogaster.
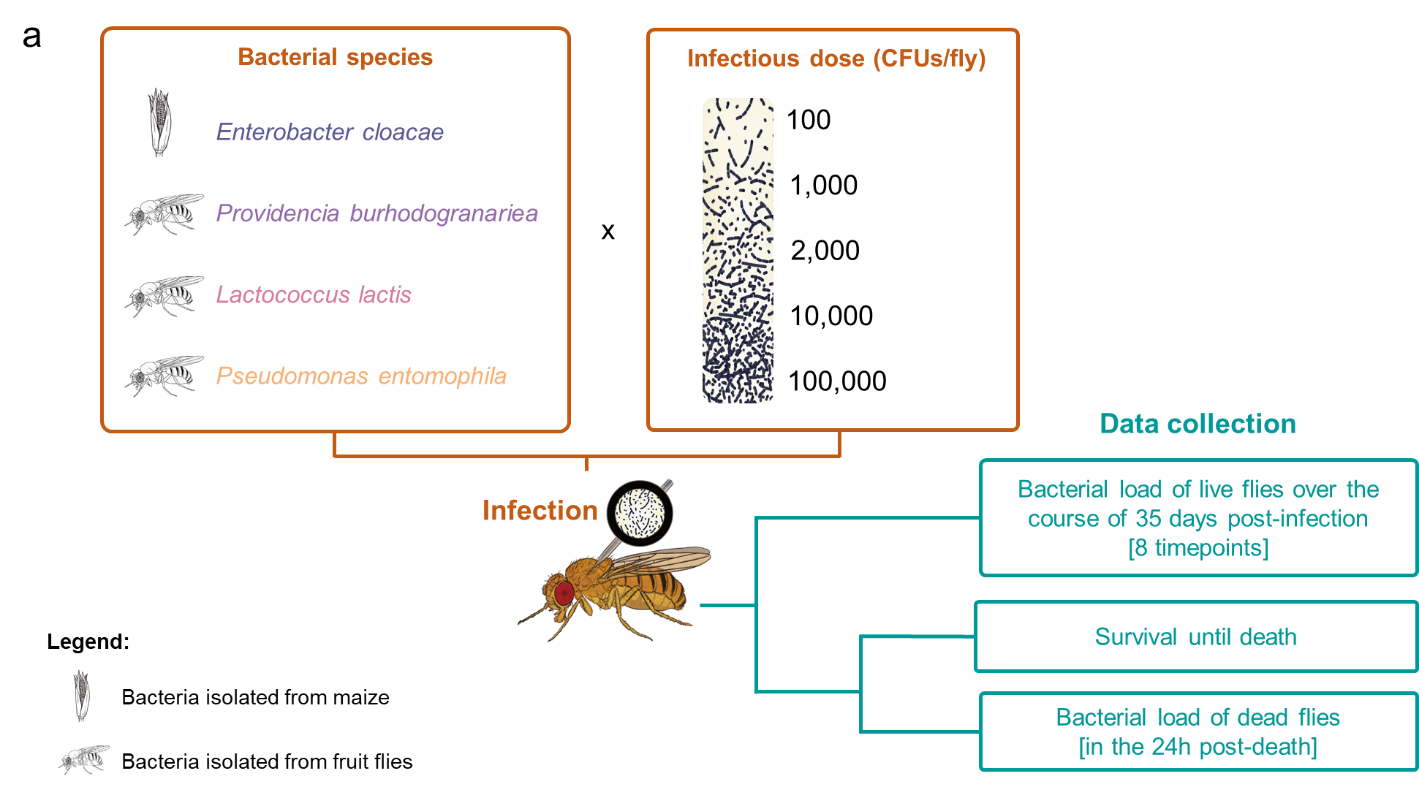
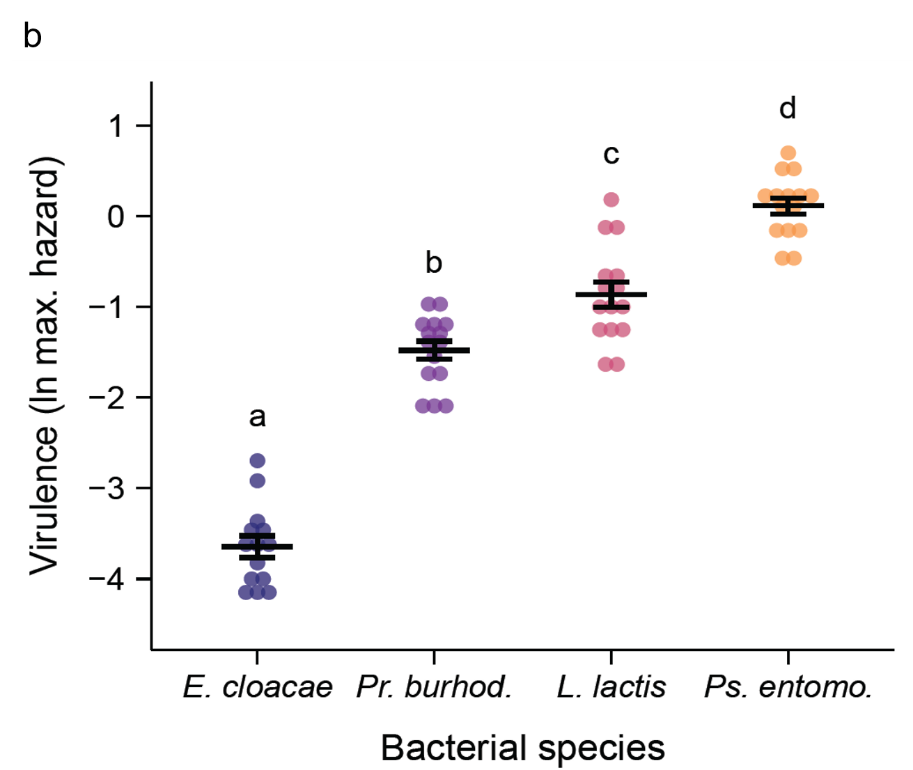
Figure 3. Experimental design and bacterial species and their virulence. a. Experimental setup: Female fruit flies were injected with one of four bacterial species, and one of five different infectious doses (in colony-forming units per fly: CFUs/fly). A first subset of fruit flies was used for data collection on bacterial load of live flies. Flies were collected and homogenised on days 1, 2, 3, 4, 7, 14, 21, 28 and 35 post-infection (amounting to 8 timepoints. A second subset of flies was used for collecting data on survival post-infection. The survival of flies was assayed every day until they died. For this subset, between the intervals 14-35- and 56-78-days post infection, we assayed the bacterial load of flies found dead during the daily survival check-up. b. Virulence measured as the natural log of maximum hazard for all bacterial species, where each data point is the maximum hazard between zero- and 20-days post injection, calculated from one experimental replicate per bacterial dose. Black lines show means and standard errors. We fit a linear model (two-tailed) where the main effect of bacterial species was p < 0.0001 (see main article).
The infecting bacterial species included three opportunistic pathogen species (i.e., Lactococcus lactis, Providencia burhodogranaeria and Pseudomonas entomophila) and a common plant bacterium, Enterobacter cloacae (Figure 3a). Bacteria were ranked from least to most virulent, measured as cost in survival to the host, from E. cloacae, Pr. burhodogranaeria, L. lactis and Ps. entomophila (Figure 3b). “Cradle to grave” infection studies are scarce and their lack drives the misconception that most infection only have two possible outcomes: survival with persistent infection or death by pathogen over-proliferation. Therefore, we decided to track infection status until death. This allowed us to confirm that all species can be found in individuals up to 75 days post infection, and that the likelihood of being cleared (i.e., clearance index) depends on not only the injection dose but also the different components of their virulence. For instance, we observed that the least and the most virulent species, E. cloacae and Ps. entomophila respectively, lead the clearance levels (Figure 4). Regarding intermediate virulence species, by decomposing virulence, we observed L. lactis was able to better exploit its host than Pr. burhodogranaeria but no differences in PPP (Figure 5). The latter suggests that the higher virulence found in L. lactis is likely due to its capacity to multiply and exploit the host, and not necessarily any difference in harm, when compared to its fellow Pr. burhodogranaeria. On the other hand, the differences in virulence observed between the two intermediate virulence species and E. cloacae, the lowest one, arise from a combination of both components, exploitation and harm to the host (PPP). Altogether, our data suggests assessing different host-pathogen properties might help understand infection outcome, as well as to identify target points for future research and treatment of infections.
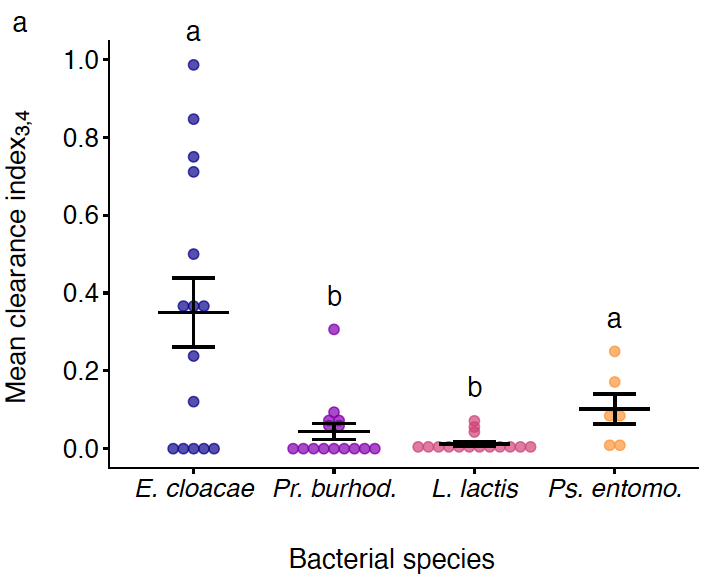
Figure 4. Clearance indexes across bacterial species. Mean species differences in clearance index for days 3 and 4 post-injection. Black lines show means and standard errors. We fit a linear model (two-tailed) where the main effect of bacterial species was p = 0.0016 (see main text). Different letters denote means that are significantly different from one another (Mann-Whitney-U post hoc tests, two-tailed tests, see main text for statistical results).
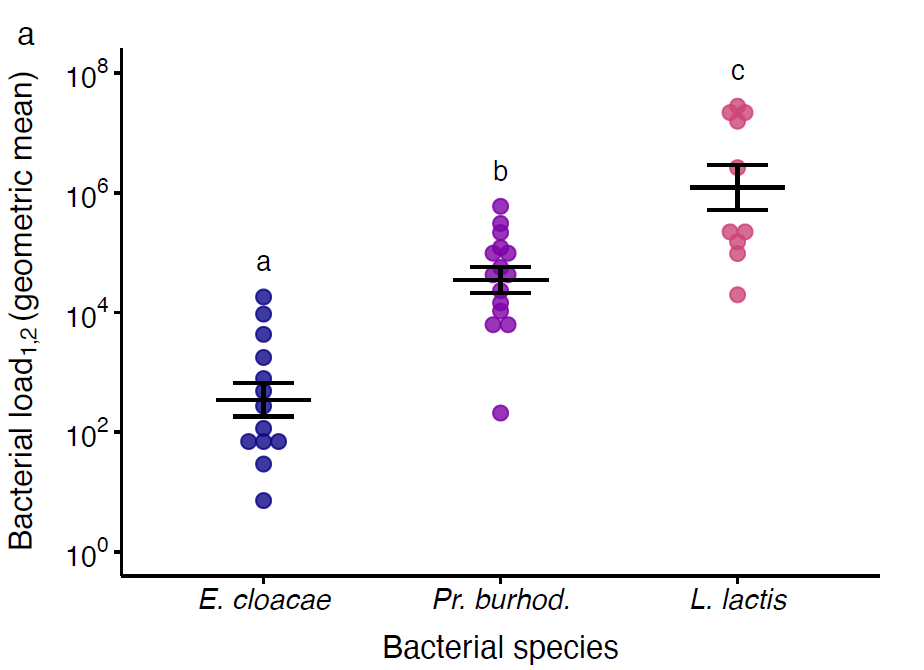
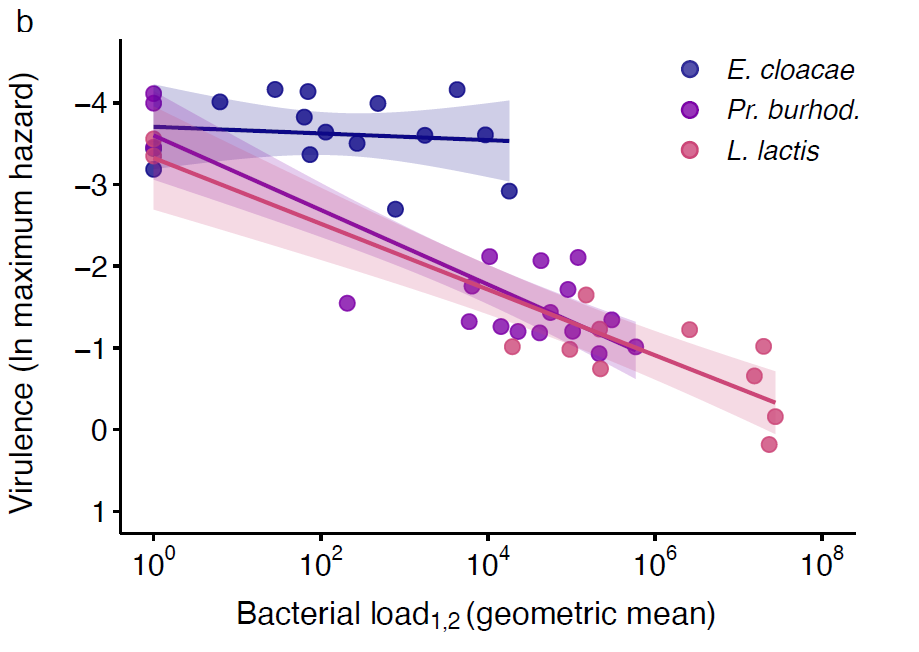
Figure 5. Pathogens’ exploitation and PPP explain their virulence. a. Parasite exploitation given as infection intensity/bacterial load across species. Each data point is one of five injection doses per bacterial species, per experimental replicate, and gives the geometric mean of bacterial load for days one and two post injection (denoted as 1,2. Black lines show means and standard errors. We fit a linear model (two-tailed) where the main effect of bacterial species was p < 0.000 (see main article). Different letters denote means that are significantly different from one another. b. PPP given as the relationship between bacterial load and the inverse of maximum hazard, so that the virulence increases with proximity to the x-axis. The bacterial load data is the same as that given in a but with the addition of the Ringer’s control group. To allow inclusion of the uninfected Ringer’s control group to the figure, we added one CFU to all mean bacterial load values. The natural log of maximum hazard data is estimated from survival data for the corresponding injection doses and experimental replicates. Lines show linear regressions with 95 % confidence intervals.
Our study shows that persistent infection and clearance are both possible outcomes of bacterial infections. More importantly, we show that decomposing virulence into exploitation and PPP provides deeper knowledge into the pathogens’ infecting strategies and can be used when not only between different genotypes but also between different infection types, in our case differing in organism origin and species. This novel application of this framework allowed us to understand which components impact clearance or survival to infection, for instance lower exploitation and PPP in E. cloacae helps to explain its low virulence compared to its counterparts (Figure 5). These tools have already started to be used with other infections and have been useful to understand their dynamics and population structure [6]. We believe the addition of clearance into a now extended framework, will surely contribute to different fields of evo-eco and biomedicine, as well as treatment development. Our data enforces that PPP and exploitation might act independently and as such, they should be considered in future infection studies. By characterizing and differentiating the source of harm to the host, we might be able to customise better care for infections in the short and long run.
References
- Graham, A. L., Allen, J. E., & Read, A. F. (2005). Evolutionary causes and consequences of immunopathology. Annual Review of Ecology, Evolution, and Systematics, 373-397.
- Medzhitov, R., Schneider, D. S., & Soares, M. P. (2012). Disease tolerance as a defense strategy. Science, 335(6071), 936-941.
- Schmid-Hempel, P. (2021). Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics. Oxford University Press.
- Råberg, L., & Stjernman, M. (2012). The evolutionary ecology of infectious disease virulence. Ecoimmunology, 548, 78.
- Kutzer, M. A., & Armitage, S. A. (2016). Maximising fitness in the face of parasites: a review of host tolerance. Zoology, 119(4), 281-289.
- Bertels, F., Marzel, A., Leventhal, G., Mitov, V., Fellay, J., Günthard, H. F., ... & Swiss HIV Cohort Study. (2018). Dissecting HIV virulence: heritability of setpoint viral load, CD4+ T-cell decline, and per-parasite pathogenicity. Molecular biology and evolution, 35(1), 27-37.
- Duneau, D., Ferdy, J. B., Revah, J., Kondolf, H., Ortiz, G. A., Lazzaro, B. P., & Buchon, N. (2017). Stochastic variation in the initial phase of bacterial infection predicts the probability of survival in D. melanogaster. Elife, 6.
Fly and bacteria illustrations by Beatriz Acuña Hidalgo
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in