How does intra-tumour low oxygen levels relate to the clinical outcome after radiotherapy in breast cancer?
Published in Cancer
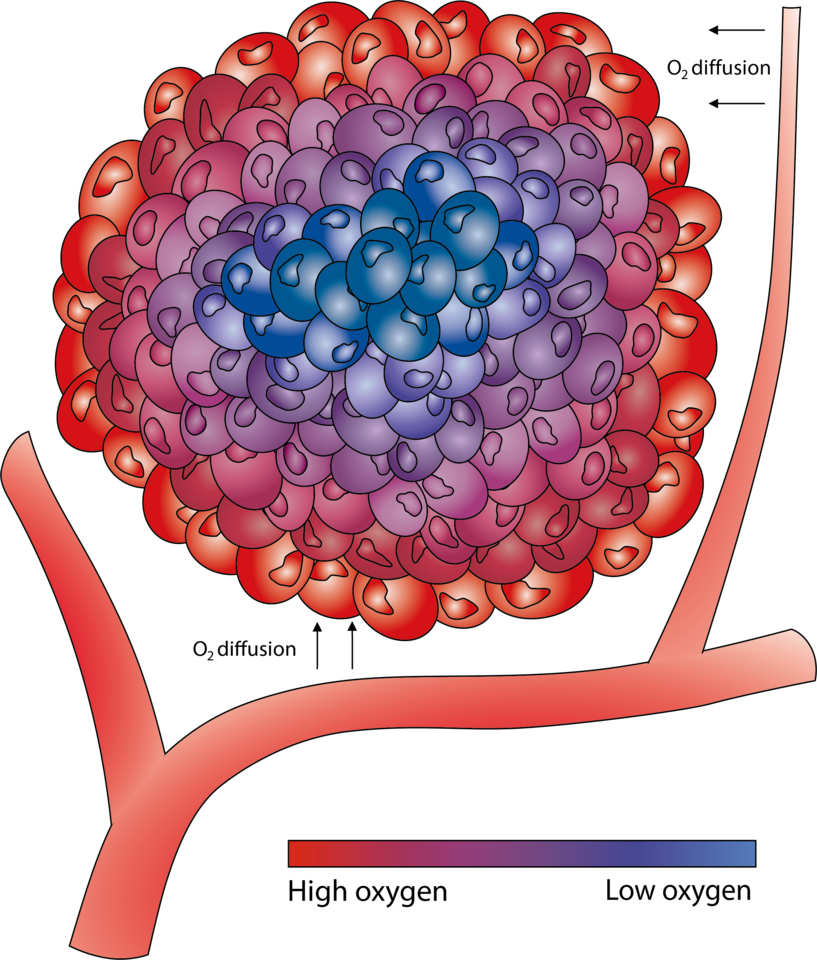
 Low oxygen levels (hypoxia) are common in cancers and have been found to correlate to worse outcome in several cancer types, including breast cancer1-3. The rapid and dense cell growth in cancer often result in the tumour outgrowing its blood supply, which leads to oxygen levels being insufficient for normal cell function and respiration, necessitating cellular adaptation. Since the discovery of the hypoxia inducible factors, HIF-1α and HIF-2α, and the elucidation of the mechanisms whereby hypoxia induces these factors to accumulate and become active transcription factors, their impact on tumour biology has been extensively studied. Two years ago, researchers behind some of these findings were awarded the Nobel prize in Physiology or Medicine.
Low oxygen levels (hypoxia) are common in cancers and have been found to correlate to worse outcome in several cancer types, including breast cancer1-3. The rapid and dense cell growth in cancer often result in the tumour outgrowing its blood supply, which leads to oxygen levels being insufficient for normal cell function and respiration, necessitating cellular adaptation. Since the discovery of the hypoxia inducible factors, HIF-1α and HIF-2α, and the elucidation of the mechanisms whereby hypoxia induces these factors to accumulate and become active transcription factors, their impact on tumour biology has been extensively studied. Two years ago, researchers behind some of these findings were awarded the Nobel prize in Physiology or Medicine.
It was shown early that tumour hypoxia could diminish the effect of radiotherapy since radiation induces reactive oxygen species that are harmful to cancer cells, but this requires adequate oxygen supply. Radiotherapy is standard of care for several cancer types. In early-stage breast cancer, postoperative radiotherapy reduces ipsilateral breast tumour recurrence and is part of the recommended treatment. However, radiotherapy comes with side effects for the patients, and there is a need of identifying patients who do not benefit and therefore can be spared treatment. The main goal of our study was to address whether tumour hypoxia is associated with low response to postoperative radiotherapy in early-stage breast cancer.
The short half-life makes HIF-1α technically difficult as a protein biomarker. Additionally, HIF-1α expression is primarily regulated at the protein level and assumed to have low concordance between mRNA expression and protein level. This makes routine evaluation of tumour hypoxia in a clinical setting challenging. Therefore, a secondary aim of this study was to evaluate the congruency of HIF-1α protein expression and gene expression signature scores that may serve as alternative markers of tumour hypoxia.
To address these questions, we studied tumour material from a unique trial that included 1187 women with lymph node-negative breast cancer that following breast-conserving surgery were randomised to receive radiotherapy or not. To stratify tumours based on their hypoxic profile we performed HIF-1α immunohistochemistry and calculated gene expression scores of 11 cancer-derived hypoxia gene signatures selected from the literature.
In this trial cohort, 1 in 5 primary tumours exhibited HIF-1α positivity4. These tumours were more likely to be high grade, hormone receptor negative, and exhibit high levels of the proliferation marker Ki67. Among triple-negative and HER2 positive tumours, 50% and 40% had high HIF-1α, respectively. HIF-1α protein levels correlated with the scores of 10 of the 11 hypoxia gene signatures. The scores generally had a high level of correlation, but the gene overlap between the signatures was relatively low. The genes that were most abundant in the studied signatures were ADM, NDRG1, SLC2A1, VEGFA, ALDOA, IGFBP3, LDHA, P4HA1, and TPI1. Notably, most of these are transcriptionally regulated by HIF-1α.
To address the relationship between hypoxia and effect of radiotherapy, we performed stratified cumulative incidence analyses and Cox regressions for interaction. In contrast to our hypothesis, no statistical difference in terms of benefit of radiotherapy in preventing local recurrences or breast cancer-specific mortality could be shown between tumours with or without HIF-1α. Thus, also women with hypoxic primary breast cancer had benefit of postoperative radiotherapy.
However, high HIF-1α protein level was an independent risk factor for developing a recurrence within 5 years after surgery, both when considering any recurrence, and for local recurrences exclusively. After adjusting for age, tumour size, subtype, and systemic adjuvant treatment, the risk of recurrences was at least 70% higher for HIF-1α positive tumours. We hypothesised that the HIF-1α status of a local recurrence would have a more prominent prognostic impact for survival than that of the primary tumour. Indeed, we found that a local recurrence with high HIF-1α were three times more likely to lead to breast cancer-specific mortality within 5 years after surgery of the recurring tumour, than a HIF-1α negative recurrence.
Several gene expression signature scores were prognostic of local recurrence and any recurrence within 5 years, as well as breast cancer-specific mortality within 10 years. Most notably, signatures presented by Buffa et al. showed a strong association to all three outcomes. Notably, no signature score outperformed immunohistochemical HIF-1α staining as a prognostic marker in our material.
Some findings of this study may illuminate new aspects of the biological behaviour of HIF-1α. Firstly, in contrast to the idea of HIF-1α protein levels being mainly regulated on the protein level rather than transcriptionally, we discovered a clear correlation between HIF1A mRNA and HIF-1α protein expression. Although the mRNA expression showed a less clear association to patient outcome and thus may be an inferior marker of biologically active HIF-1α, this finding suggests that transcriptional activity of HIF1A has a non-negligible impact on HIF-1α protein expression.
The results of this study falsified the main hypothesis, that cancer cells seeded from more hypoxic primary breast tumours would be more resistant to radiotherapy. This finding is in contrast with findings in some other cancer types, including head and neck tumours5. In our material, radiotherapy was applied after surgery and wound healing. In this setting, irradiation is aimed to eliminate any remaining cancer cells seeded from the removed primary tumour. In some other tumour types and stages, radiotherapy is applied prior to surgery or as a therapy without surgery. In these cases, environmental factors such as tumour infiltrating cells and associated vessels remain and are subjected to inflammation and tissue repair mechanisms. It is likely that the influence of tumour hypoxia on the effect of radiation differs between these two situations. Interestingly, data from paired primary tumours and local recurrences revealed that the HIF-1α status to a high degree seemed to be inherited from the primary tumour to the recurrence – even when the latter developed years after primary tumour resection. This suggests that HIF-1α status not only is a response to an existing intra-tumour microenvironment, but that there is a remaining predisposition for developing a hypoxic tumour – either encoded in the genetics of remaining tumour cells, or a lingering factor in the microenvironment or host.
To summarise, in samples from a large breast cancer trial we found HIF-1α to be a prognostic marker for outcome of early-stage breast cancer, but not predictive for radiotherapy response. Several hypoxia-related gene expression signatures were found to be prognostic of recurrences and breast cancer-specific mortality and may be considered as prognostic tools for early-stage breast cancer. HIF-1α protein staining remained the strongest hypoxia-related prognostic marker of recurrence and breast cancer-specific mortality.
- Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, et al. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97(6):1573-81.
- Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield S, et al. Hypoxia-inducible factor-1alpha expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin Cancer Res. 2006;12(15):4562-8.
- Jögi A, Ehinger A, Hartman L, Alkner S. Expression of HIF-1alpha is related to a poor prognosis and tamoxifen resistance in contralateral breast cancer. PLoS One. 2019;14(12):e0226150.
- Tutzauer J, Sjostrom M, Holmberg E, Karlsson P, Killander F, Leeb-Lundberg LMF, et al. Breast cancer hypoxia in relation to prognosis and benefit from radiotherapy after breast-conserving surgery in a large, randomised trial with long-term follow-up. Br J Cancer. 2022.
- Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res. 2016;57 Suppl 1:i90-i8.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in