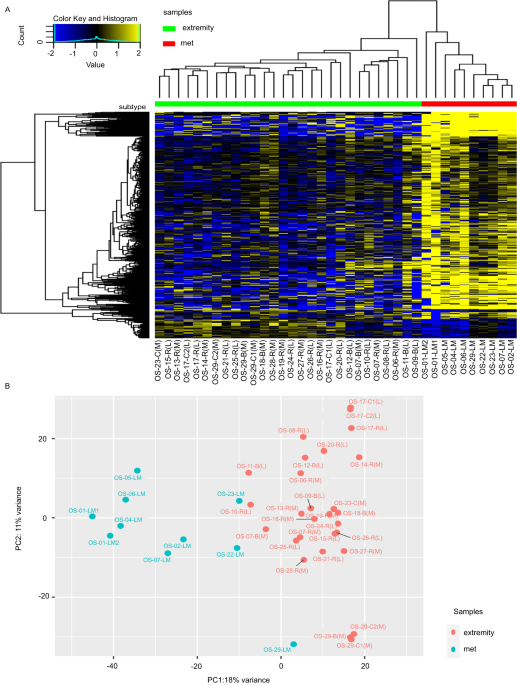
Although considered a rare cancer, osteosarcoma (OS) is the most common primary bone tumor1. Approximately 1,000 people are diagnosed with OS in the United States each year2. Unfortunately, most patients are between 10 and 19 years of age3. Standard-of-care protocols of chemotherapy and surgical intervention have yielded a 5-year survival rate of 64 – 76% for localized disease. However, outcomes for patients with metastatic OS are much more dismal with 5-year survival rates of less than 30%4. We recently published our study with Oncogene which compared the RNA sequences of primary OS tumors and metastatic OS tumors from the lung (the primary site of metastasis). Our research demonstrated similarities and differences between primary and metastatic OS tumors. We also explored the effects of targeting aldehyde dehydrogenase 1A1 (ALDH1A1) and the androgen receptor (AR) in OS in vitro as these proteins stood out from our pathway analysis of lung metastases (LM).
We are proud to share our work and hope that it will be helpful to the Oncogene community. Please remember that our sequencing data is public with NCBI’s Gene Expression Omnibus if you are interested in exploring these data further. With the help of our clinical partners, we were able to evaluate the differential gene expression from ecRNA-seq data of OS patient tumor samples and analyze these sequences based on their disease progression. OS LM responds differently to treatment and therefore has very different outcomes compared to primary and recurrent OS cases. Our Oncogene article reveals that the genomic signatures of OS LM are less similar to their matched primary than to other unmatched OS LM. For decades clinicians, patients, and researchers of OS have known that OS LM does not behave like primary OS, but little progress has been made to elucidate why this may be. Our study provides insight into the genomic makeup of OS and highlights the importance of LM in this cancer.
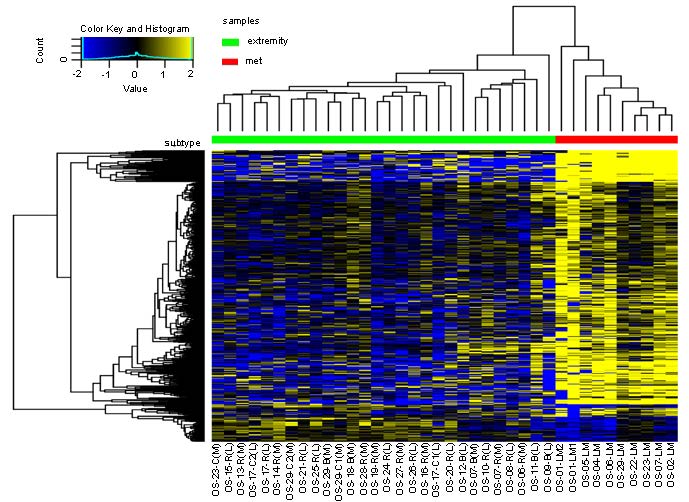
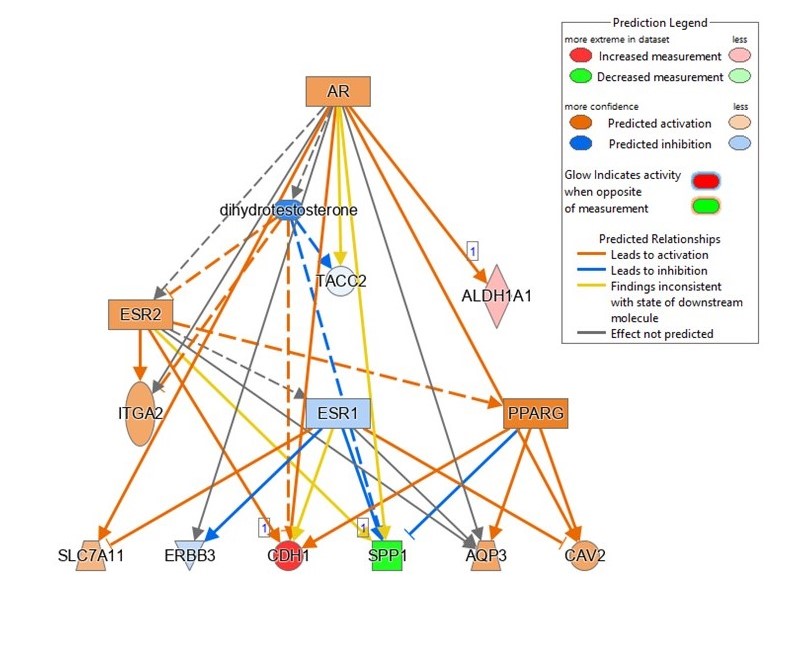
For years our lab, the Musculoskeletal Oncology Lab (MOL), has investigated the involvement of the stem cell marker ALDH1A1 in OS LM5-7. In our most recent publication, we saw significant increases in ALDH activity and expression in both our RNA-seq analysis and a series of in vitro experiments. Interestingly, pathway analyses of our 38 OS tumor samples (28 unique patients) predicted ALDH1A1 directly downstream from the activation of androgen receptor (AR), and ALDH1A1 was upregulated in OS LM samples. Because OS is prevalent in children and young adults at the peak of their growth velocities, AR has been the topic of several OS research hypotheses. However, because of the rarity of OS and the difficulty of obtaining appropriate samples, studies have been limited. A cohort of 28 patients may seem small, but the incidence of OS in the U.S. is so low that our sample size is equivalent to a cohort of over 8 thousand patients in a breast cancer study (with an incidence of 313,510 predicted cases per year)8. We are confident with the data presented in this work and look forward to building upon our discoveries.
The MOL is uniquely situated for the development of a program that collects fresh specimens from patients with OS and other sarcoma types, all of which are rare tumors. We have thus been collecting a series of fresh tissue and matched blood samples from surgical candidates at the University of Pittsburgh Medical Center (UPMC) since 2012. The resulting biobank is extremely valuable in furthering our work to understand and improve the outcomes of OS LM. While our focus remains on the treatment and improvement of outcomes for patients with OS LM, we understand the value of collecting all sarcoma subtypes and metastatic bone carcinoma tumors. We believe that collaboration is essential to study rare cancers and we hope that our biobank can be an integral part of future collaborative sarcoma research.
References
1. Misaghi, A., Goldin, A., Awad, M., & Kulidjian, A. Osteosarcoma: a comprehensive review. SICOT J. 4, 12 (2018).
2. Siegel, R.L., Giaquinto, A., & Jemal, A. Cancer statistics, 2024. CA: A Cancer Journal for Clinicians. 74, 12-49 (2024).
3. Williams, L.A. & Spector, L.G. Survival differences between males and females diagnosed with childhood cancer. JNCI Cancer Spectr. 3(2), pkz032 (2019).
4. Wessel, M. et al. (2023, March 1). Survival Rates for Osteosarcoma. American Cancer Society. Retrieved February 5, 2024, from https://www.cancer.org/cancer/types/osteosarcoma/detection-diagnosis-staging/survival-rates.html
5. Mu, X. et al. Rapamycin inhibits ALDH activity, resistance to oxidative stress, and metastatic potential in murine osteosarcoma cells. Sarcoma. 480713 (2013).
6. Mu, X et al. Retinal targets ALDH positive cancer stem cell and alters the phenotype of highly metastatic osteosarcoma cells. Sarcoma. 784954 (2015).
7. Mandell, J. et al. ALDH1A1 Gene Expression and Cellular Copper Levels between Low and Highly Metastatic Osteosarcoma Provide a Case for Novel Repurposing with Disulfiram and Copper. Sarcoma. 7157507 (2022).
8. American Cancer Society. (Jan 2024). Key Statistics of Breast Cancer. https://www.cancer.org






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in