Huntingtin’s new interaction site: a tale of DNAJB1 and HTT’s proline-rich domain
Published in Healthcare & Nursing
Huntington´s disease (HD) is a hereditary neurodegenerative disorder caused by a mutation in the protein huntingtin (HTT). HTT is a large ubiquitous protein of 3144 amino acids that is processed by caspases resulting in smaller fragments, with the functions of these fragments being highly unknown. In HD, the first fragment (HTTExon1) has an extended sequence of glutamine (Q) amino acids, which causes HTT to aggregate and leads to neurotoxicity 1 2. The extended polyQ region in HTTExon1 is responsible for forming amyloid fibrils that can be detected in neurons of HD patients 3,4. The formation of amyloid fibrils is further regulated by the polyQ-flanking domains, the N17 and the C-terminal proline-rich domain (PRD) 5–7.
The research objective here was to determine how molecular chaperones may regulate amyloid fibril formation, potentially by interacting with the N17 or PRD regions within HTTExon1. The starting point of this research project was our previous observation that a trimeric chaperone complex can interact with the soluble Huntingtin protein to suppress its aggregation into amyloid fibrils, and can further disaggregate HTT amyloid fibrils 8. This trimeric chaperone complex is composed of Hsc70, a J-domain protein (JDP) DNAJB1 and the nucleotide exchange factor Apg2.
The observation that the same chaperone complex can interact with soluble as well as aggregated HTTExon1 suggested that the binding interface could be the same in both folding states. We knew that all three chaperones are required for the remodeling of HTTExon1. However, we did not know if all three chaperones bind simultaneously to form a physical complex with HTT or whether HTT is bound to each component in series and transferred between the chaperones. We set out to first identify which chaperones directly interact with HTT and then to uncover the binding interface between HTT and the chaperones using crosslink mass spectrometry, creating bonds between HTT and the chaperones before measuring the fragment sizes using mass spectrometry.
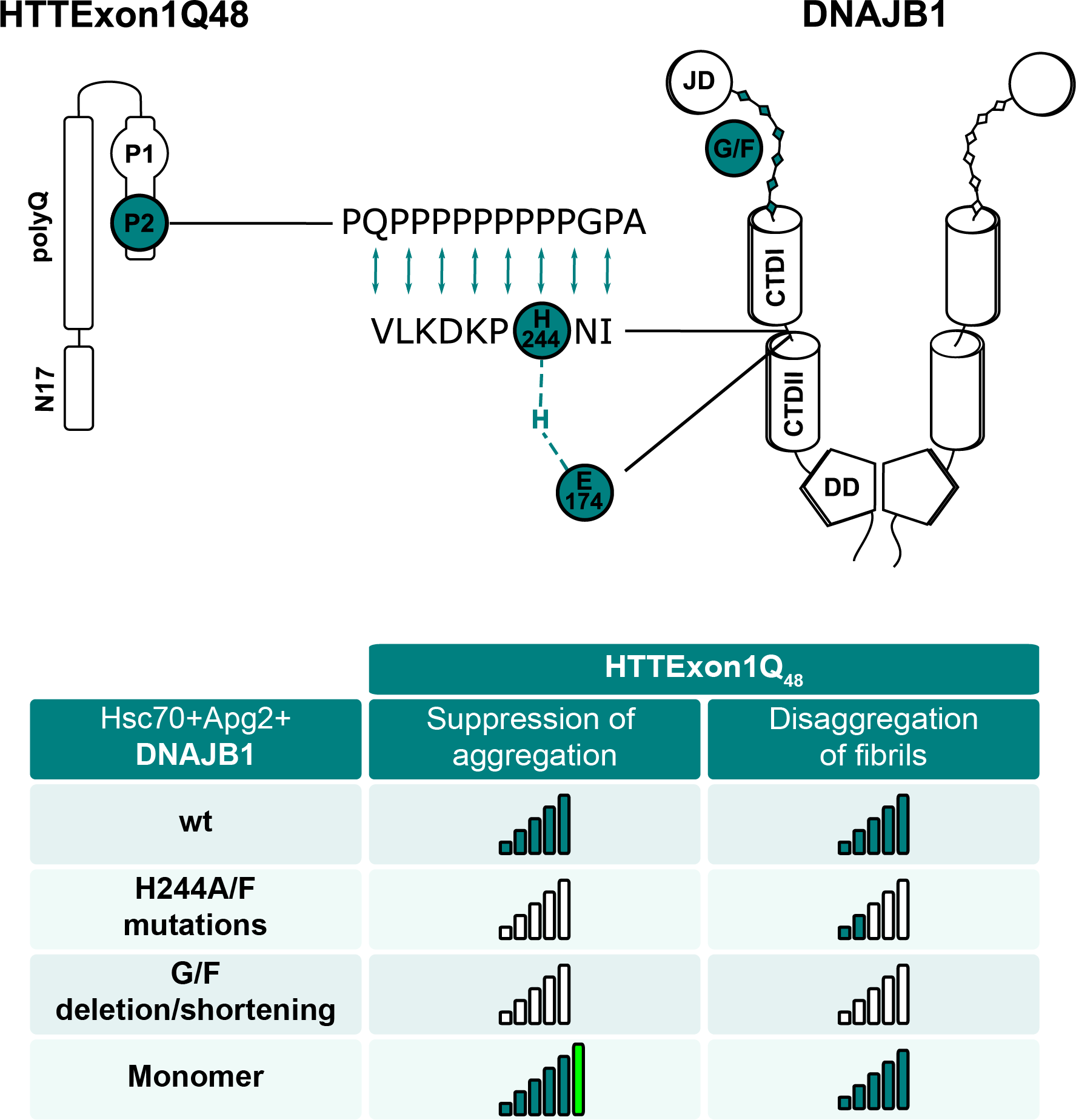
Most conventional crosslinkers react with primary amines such as lysine and hence reduce the analysis of the potential interaction interface within HTT to the only stretch of the protein that contains lysine residues, the N17 domain. To overcome this limitation, we used a non-specific crosslinker as well as a non-specific protease. Interestingly, we identified an interaction of HTTExon1 with both, Hsc70 and DNAJB1, when all chaperones were present. We did however identify an interaction between HTTExon1 and DNAJB1 also in the absence of the other chaperones. No interaction was detected between HTTExon1 and Apg2.
Surprisingly, HTTExon1 interacts with DNAJB1 (and Hsc70) via its PRD. The binding site within DNAJB1 mapped to nine amino acids (aa) of the hinge region between the C-terminal domains I and II. Did we identify a new motif for the recognition of HTT? We noticed that a histidine residue was 100% conserved among related J-domain proteins. When the histidine is mutated to alanine, HTTExon1 fibrils began to form again. Importantly, this mutation had no effect on any other tested chaperone substrate. We concluded first that this histidine at position 244 (His244) is required for the suppression of HTTExon1 aggregation and second that it is specific for HTT.
What is the role of His244? To address this question, we performed molecular dynamics simulations and docking analyses. There were challenges to developing these molecular models, since there are unstructured regions in HTTExon1 that needed to be explored through advanced simulation techniques. The structural ensemble created were used to develop docked models to study interactions between DNAJB1 and HTTExon1. These in silico models showed that His244 forms a hydrogen bond with a glutamate residue within the hinge region of DNAJB1. Forming this hydrogen bond, and other interactions identified in simulation, likely stabilize the binding interface between DNAJB1 and HTTExon1, thereby preventing aggregation.
What puzzled us was the fact that His244 was also conserved in other JDPs such as DNAJA1 that did not show any chaperone activity towards HTT with Hsc70 and Apg2. Clearly, there must be other properties within the J-domain protein that are directly or indirectly involved in the interaction with HTT. When comparing the domain architecture of DNAJA1 and DNAJB1 two main differences became apparent: 1. The linker region that connects the J-domain to the C-terminus is longer in DNAJB1 and 2. DNAJA1 harbors a Zinc-finger like region (ZFLR) that is absent in DNAJB1. We systematically deleted and swapped domains between these two J-proteins. In the end, we could functionalize DNAJA1 towards HTT by replacing its linker with the longer linker of DNAJB1. This chimeric protein could substantially delay, but still not completely suppress the aggregation of HTT together with Hsc70 and Apg2. Thus, there must be additional features within DNAJB1 that contribute to the binding and maybe also transfer of HTT to Hsc70 in the chaperone cycle.
What about the binding to HTT fibrils and its disaggregation? Structural analyses of HTTExon1 fibrils placed the PRD of HTT outside of the fibril core and hence accessible for chaperones 9. HTT fibrils that lacked the identified proline stretch could not be bound by DNAJB1 anymore and thus did not disaggregate. Mutating His244 reduced the affinity to HTT fibrils and consequently also strongly diminished the ability of the trimeric chaperone complex to disaggregate HTT fibrils. However, the single point mutant was not completely inactive towards fibrils as for the soluble HTT. Thus, the chaperone activities of suppressing soluble HTT and disaggregating HTT fibrils share binding sites within DNAJB1 such as His244, but use additional yet unknown sites within DNAJB1 or in the subsequent steps of HTT transfer to Hsc70.
Although many open questions remain, we could contribute to the understanding how a chaperone generalist such as DNAJB1 that can interact with a wide range of proteins can discriminate and specifically bind a substrate protein and even in two very different conformations.
Published article: https://rdcu.be/cTo3B
References:
- A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 72, 971–983 (1993).
- Graham, R. K. et al. Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant huntingtin. Cell 125, 1179–1191 (2006).
- Davies, S. W. et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90, 537–548 (1997).
- DiFiglia, M. et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997).
- Bhattacharyya, A. et al. Oligoproline effects on polyglutamine conformation and aggregation. J. Mol. Biol. 355, 524–535 (2006).
- Dehay, B. & Bertolotti, A. Critical role of the proline-rich region in Huntingtin for aggregation and cytotoxicity in yeast. J. Biol. Chem. 281, 35608–35615 (2006).
- Pigazzini, M. L., Lawrenz, M., Margineanu, A., Kaminski Schierle, G. S. & Kirstein, J. An Expanded Polyproline Domain Maintains Mutant Huntingtin Soluble in vivo and During Aging. Front. Mol. Neurosci. 14, 721749 (2021).
- Scior, A. et al. Complete suppression of Htt fibrilization and disaggregation of Htt fibrils by a trimeric chaperone complex. EMBO J. 37, 282–299 (2018).
- Isas, J. M., Langen, R. & Siemer, A. B. Solid-State Nuclear Magnetic Resonance on the Static and Dynamic Domains of Huntingtin Exon-1 Fibrils. Biochemistry 54, 3942–3949 (2015).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in