Hydrogen-bonded Framework Mimics Combustible Ice Behavior at Ambient Condition
Published in Chemistry

Natural gas hydrate, also termed as combustible ice, generally distributes in abyssal sediment and permafrost. As a solid bulk, combustible ice of 1 m3 can decompose into 164 m3 CH4 and produce only CO2 and H2O upon combustion, leaving no solid residues or harmful gases, which make it ideal clean energy.
It is however noteworthy that the greenhouse effect induced by CH4 in combustible ice is estimated to be 20-fold of CO2. One can imagine that if the CH4 in combustible ice escapes from seafloor to ambient environment, what a dangerous world will be! The existing exploitation methods are of high cost and low efficiency, mainly because that the formation of combustible ice requires low temperature (0-10 oC) and high pressure (>30 atm), elevated temperature or decreased pressure will result in CH4 release and decomposition of solid hydrate.
We anticipate that if these processes can be completed in artificially synthesized materials at ambient condition, the reversible adsorption (storage) and release of guest molecules will make great sense in both science and practice. Most reported porous materials are able to capture guest molecules when serving as adsorbents, but the release of guest molecules generally requires extra energy input. Hydrogen-bonded organic frameworks (HOFs) featuring moderate strength of hydrogen bonds drew our attention in terms of their structural flexibility, despite of the fragile framework at ambient condition.
We envisioned that metastable HOFs will collapse and simultaneously release guest molecules, while the reconstruction of the framework accompanied with re-adsorption of guest molecules can be realized at mild condition. To this end, we found that the ionic HOF ([B(OCH3)4]3[C(NH2)3]4Cl•4CH3OH, termed Gd-B) constructed from guanidinium cation and borate anion at ambient condition, via electrostatic interaction and hydrogen bonding. When freshly-prepared Gd-B is exposed in air atmosphere, all sixteen methanol molecules are released via desorption and tetramethyl borate hydrolysis, the amount reaches high up to 60 wt% of Gd-B crystal. At room temperature and atmospheric pressure, interestingly, the decomposed product can transform into Gd-B when exposed in methanol atmosphere (liquid or vapor), along with the re-adsorption of guest methanol molecules confined in the pores of Gd-B. All these processes automatically proceed and no extra energy input (such as heating, vacuum, etc.) is of necessity. Finally, we also detected that the guest methanol stored in Gd-B can be released in air and further ignited, leaving the main structure unaltered.
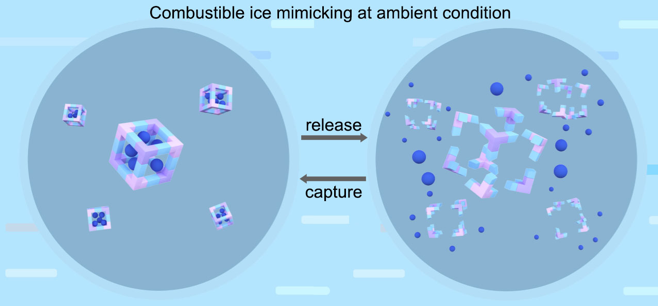
Figure 1. Schematic image of reversible structural transformation and release/capture of guest molecules in HOFs
Although weaker intermolecular interactions (H-bond, Van der Waals, and electrostatic forces, etc.) usually lead to metastable porous frameworks, they bring new opportunities for reversible release/adsorption of guest molecules in moderate fashion, without extra energy input to eliminate the guest–host interactions. These porous frameworks that mimic combustible ice at ambient condition, will benefit energetic material unitization, moisture capture/release in a facile and economic way.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in