Hydroxylamine-Mediated C-C Amination via an Aza-Hock Rearrangement
Published in Chemistry
Anilines, as key units, are vitally important to pharmacy, agrochemistry, and organic chemistry. Such motifs are typically accessed by the traditional nitration/reduction sequence or by cross-coupling reactions and modern methods for direct arene C-H aminations. Alternatively, direct C-C amination is a good choice to address the site-selectivity problem introduced by the above methods. However, only a few cases are developed to access to anilines under metal-free conditions: one typical example via Beckmann rearrangement to anilines from ketones is improved by Uchida;1 the other typical cases of Schmidt-like rearrangement for aniline synthesis direct from benzyl alcohols/alkyl arenes with azide compounds are developed by Ning’s group.2, 3
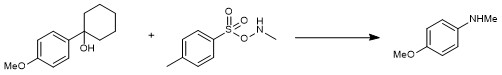
Figure 1. Initial entry of the C-C amination
However, safe and easy-to-handle protocols are still needed for direct C-C aminations. Our group presents a metal-free arene C-H amination using arylsulfonyl hydroxylamines (ArSO2ONHR) in HFIP,4 the experience of which makes us believe ArSO2ONHR could be also amenable in C-C amination. First entry using 1-(4-methoxyphenyl)cyclohexan-1-ol and N-methyl-O-tosylhydroxylamine (TsONHMe), delivered the 4-methoxy-N-methylaniline rather than the expected product of a alpha-cleavage of the cyclohexyl ring, 1-(4-methoxyphenyl)-6-(methylamino)hexan-1-one (Figure 1). Following, other secondary/tertiary benzyl alcohols could also be converted into anilines with same strategy. All this makes us believe this C-C amination (so-called aza-Hock rearrangement, Figure 2b, 2c) might go such a rearrangement similar to Hock rearrangement (Figure 2a). We supposed that the electrophilic precursor benzyl alcohol could react with nucleophilic TsONHMe to give the reactive benzyl hydroxylamine intermediate, which might undergo an aza-Hock rearrangement, then hydrolysis of the iminium intermediate delivers valuable anilines.
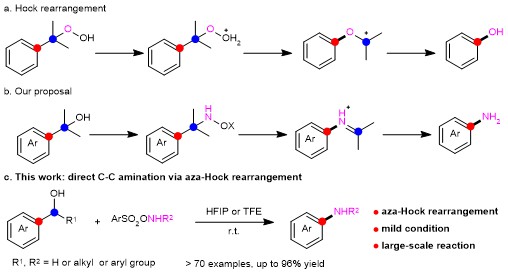
Figure 2. Hock rearrangement and our work of aza-Hock rearrangement
The scope of this C-C amination is striking, and almost all tested benzyl alcohol partners were transformed into anilines in excellent chemoselectivity: not only simple benzyl alcohols but also pharmaceutical and natural products are feasible in the aza-Hock rearrangement; primary/secondary anilines can be afforded with different aminating reagents (Figure 3).
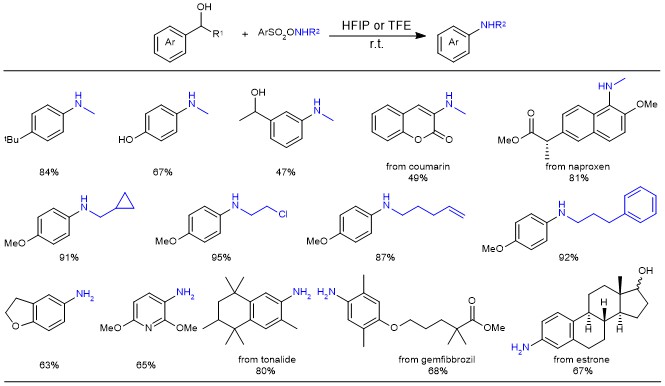
Figure 3. Substrate/hydroxylamine scope in the synthesis of anilines
Concerning the mechanism, we initially considered that a radical pathway might operate, which would be related to our previous work (direct arene C-H amination).4 However, EPR, radical trapping and radical clock experiments, attempting to trap or detect radicals, failed. We observed some bathochromic shifts (250 – 350 nm) on UV with different alcohols and TsONHMe in HFIP, but no literature precedence indicated that these shifts belong to aryl radicals. Following mechanistic studies, isolated tertiary aniline, benzyl ether and benzaldehyde unambiguously proved this C-C amination is Hock-like rearrangement rather than radical reaction. Accordingly, we propose that the reaction proceeds via an aza-Hock rearrangement in four steps: generation of a benzyl cation via benzyl alcohol solvolysis by HFIP; formation of a reactive O-(1-phenylethyl)hydroxylamine, which gives access to an iminium tosylate salt after aryl migration, and finally the irreversible hydrolysis of the imine, yielding the desired aniline after simple workup (Figure 4).
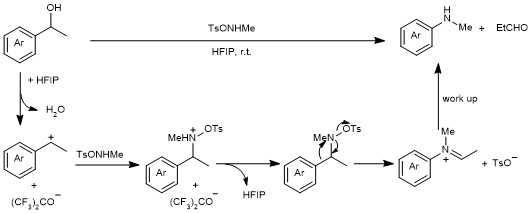
Figure 4. Proposed mechanism of the C-C aza-Hock rearrangement
Here we present a mild, general and scalable, chemoselective method for C-C aminations, delivering primary or secondary anilines in good to excellent yields. More details of this work could be found here: “Hydroxylamine-Mediated C-C Amination via an Aza-Hock Rearrangement” in Nature Communications (https://doi.org/10.1038/s41467-021-27271-y).
For other related works by our group, please see our group website: https://www.hashmi.de
References
- Hyodo, K., Hasegawa, G., Maki, H. & Uchida, K. Deacetylative amination of acetyl arenes and alkanes with C-C bond cleavage. Org. Lett. 21, 2818-2822, (2019).
- Liu, J. et al. From alkylarenes to anilines via site-directed carbon-carbon amination. Nat. Chem. 11, 71-77, (2019).
- Adeli, Y. et al. Electrochemically oxidative C-C bond cleavage of alkylarenes for anilines synthesis. ACS Catal. 9, 2063-2067, (2019).
- Wang, T. et al. A Metal-Free Direct Arene C-H Amination. Adv. Synth. Catal. 363, 2783-2795, (2021).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in