IL-24 improves efficacy of CAR-T cell therapy by targeting stemness of tumor cells
Published in Anatomy & Physiology
Cancer stem cells (CSCs) induce therapeutic resistance and may be an important barrier to cancer immunotherapy. Interleukin (IL)-24 induced apoptosis in CSCs and contributed to T cell activation, differentiation, and proliferation. IL-24 producing chimeric antigen receptor T (CAR-T) cells inhibited CSC enrichment and exhibited stronger antitumor activity in vitro and in vivo.
The problem
CSCs are self-renewable cell types that contribute to tumor onset, progression, recurrence, and metastasis1. CSCs have also been implicated in the development of treatment resistance. CSCs are dynamically enriched during disease progression and treatments. Therefore, it is a significant challenge to eliminate CSCs.
Although CAR-T cells have achieved tremendous success in the treatment of hematological malignancies, the limited clinical benefit of CAR-T cell therapy has been demonstrated in solid tumors. Cytokines, chemokine receptors, costimulatory signals, and metabolic reprogramming have been used to improve the therapeutic efficacy against solid tumors2. Metabolic engineering can enhance CAR-T cell proliferation and therapeutic activity. These strategies improve the proliferation, infiltration, and therapeutic activity of CAR-T cells3. However, these strategies majorly focus on T cell and tumor microenvironment; thus, the importance of CSCs in CAR-T cell therapy has been largely ignored.
IL-24, a member of the IL-20 subfamily of cytokines, is down-expression in tumors. Several studies have shown that IL-24 can specifically induce apoptosis of tumor cells through multiple pathways4. Moreover, chemotherapeutic- and antibody-mediated killing is enhanced in the presence of IL-24 and provides synergistic effects. It is noteworthy that IL-24 is not only nontoxic to normal cells but can also kill both unsorted cancer cells and enriched CSCs, providing further support that IL-24 may be a safe and effective method for cancer therapy5. However, the application of IL-24 in tumor immunotherapy requires further investigation.
The solution
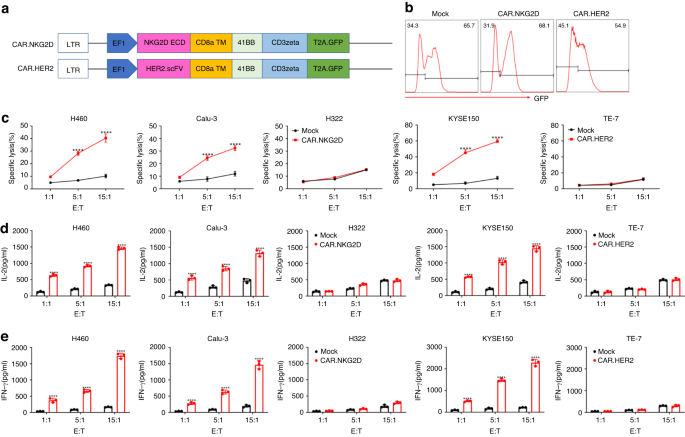
Our team designed IL-24-secreting CAR-T cells using a tandem construct encoding a second-generation CAR and IL-24 linked with a self-cleavable 2A peptide. Using conventional CAR-T cells as controls, we found that the IL-24-secreting CAR-T cells exhibited superior efficacy in lung cancer and esophagus cancer models.
We first established human IL-24-secreting CAR-T cells that target three different mouse transplanted tumor models: tail-vein injection lung metastasis cancer model, subcutaneous lung cancer and esophagus cancer models. In all models tested, the human IL-24-secreting CAR-T cells induced CSC apoptosis and augmented CAR-T cell function, leading to potent and persistent tumoricidal effects.
Detailed analyses showed that lower CD133 expression in tumor cells was observed in IL-24-secreting CAR-T cell-treated group. Meanwhile, enhanced infiltration of CD3+ T cells in tumors was found in IL-24-secreting CAR-T cell-treated group compared to the other groups, and infiltrated IL-24-secreting CAR-T cells exhibited higher IFN-g production and lower PD-1 expression. In addition, IL-24 secretion also promoted T cell memory formation, leading to durable antitumor responses and rapid tumor clearance upon rechallenge.
The implications
In fact, many cytokines have multifaceted functions and should be investigated. Insights into the complex functions of cytokines could lead to further biomedical applications. The IL-24-secreting CAR T cell described here can potentially be a generalizable approach to prevent T cell exhaustion and inhibit stemness of tumor cells. Extension to other T cell-based therapeutics such as T cell receptor-engineered T cells and tumor-infiltrating lymphocytes can be expected.
- Henkin RI. Clinical and Therapeutic Implications of Cancer Stem Cells. The New England journal of medicine. 2019;381(10):e19.
- Zhao Y, Chen J, Andreatta M, et al. IL-10-expressing CAR T cells resist dysfunction and mediate durable clearance of solid tumors and metastases. Nature Biotechnology. 2024.
- Si X, Shao M, Teng X, et al. Mitochondrial isocitrate dehydrogenase impedes CAR T cell function by restraining antioxidant metabolism and histone acetylation. Cell Metabolism. 2024;36(1):176-192.e110.
- Hu Q, Zhang Y, Wang P, et al. IL-24 armored CAR19-T cells show enhanced antitumor activity and persistence. Signal Transduct Target Ther. 2021;6(1):14.
- Cunningham CC, Chada S, Merritt JA, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11(1):149-159.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in