Imaging small molecules in cells
Published in Protocols & Methods

The cell is a wondrous 'bag' full of chemical reactions, orchestrated in an astounding and carefully controlled manner. A chemist can only dream about controlling such a plethora of reactions in a flask. Understanding life is about understanding the underlying physical and chemical processes, ranging from the electron to macromolecules. Biochemistry is a discipline trying to map the reactions in a cell, whereas chemical biology is a discipline employing chemical tools and understanding chemical reactivity in cells to decipher the hidden wonders of the living world1. Here, I am not trying to give a full picture of what chemical biology is about or what has been done by numerous colleagues over the years, but rather present you our perspective and contribution to science.
We as a group at the Curie Institute/ Paris led by Prof. Rodriguez use Chemical Biology to understand and potentially (therapeutically) exploit chemical processes in cells in disease settings, with a focus on cancer and inflammation.
Where molecules go in the cell is critical for their mechanism of action
Imagine your future husband lives in Prague and you in Paris. Unless you both never meet, this rests hypothetical. The same applies to small molecules in cells. Even if they are good binders/ interactors with a target molecule (a nucleic acid or protein for example), they have to meet. Cells are highly compartmentalised and a small molecule or drug will go depending on its biochemical properties, not because its will be 'directed' by a molecule it could interact with. Our group exemplified this in 2015 with a study on the natural product marmycin A2. This molecule is based on anthraquinone, a moiety reported to interact with DNA. However, using fluorescence microscopy we found that marmycin A accumulates in lysosomes and not the cell nucleus. Thus, studying where molecules go in cells gives important clues to their mechanisms of action. In a recent paper, a molecule based on Marmycin A was designed, adding a linker and an iron-activating moiety. This molecule can selectively kill cells with a high lysosomal iron load, including persister cancer cells. This molecule can selectively kill persister cancer cells in fresh biopsies of human tumours.
Click chemistry to visualise small molecules in cells- examples of mechanistic discoveries
Marmycin A is fluorescent but most small molecules are not and they have to be adapted to be detected in cells. One strategy is to label them with fluorophores. However, adding fluorophores directly to a small molecule, which are often bigger than the molecule itself, can change their cellular localisation. One trick is to introduce a small pretty inert chemical group on a small molecule such as an alkyne. This can then be used to react fluorophores onto the molecule after cell fixation, thus, limiting the influence on cellular localisation. The Nobel Price in Chemistry 2022 was awarded to Carolyn R. Bertozzi, Morten Meldal and K. Barry Sharpless. We extensively reviewed this approach in Nature Reviews Chemistry3. We identified salinomycin to target lysosomes in a study in 20176. Below an example of our recent publication in Nature, showing that the metformin derivative supformin accumulates in mitochondria (labelled with cytochrome c).
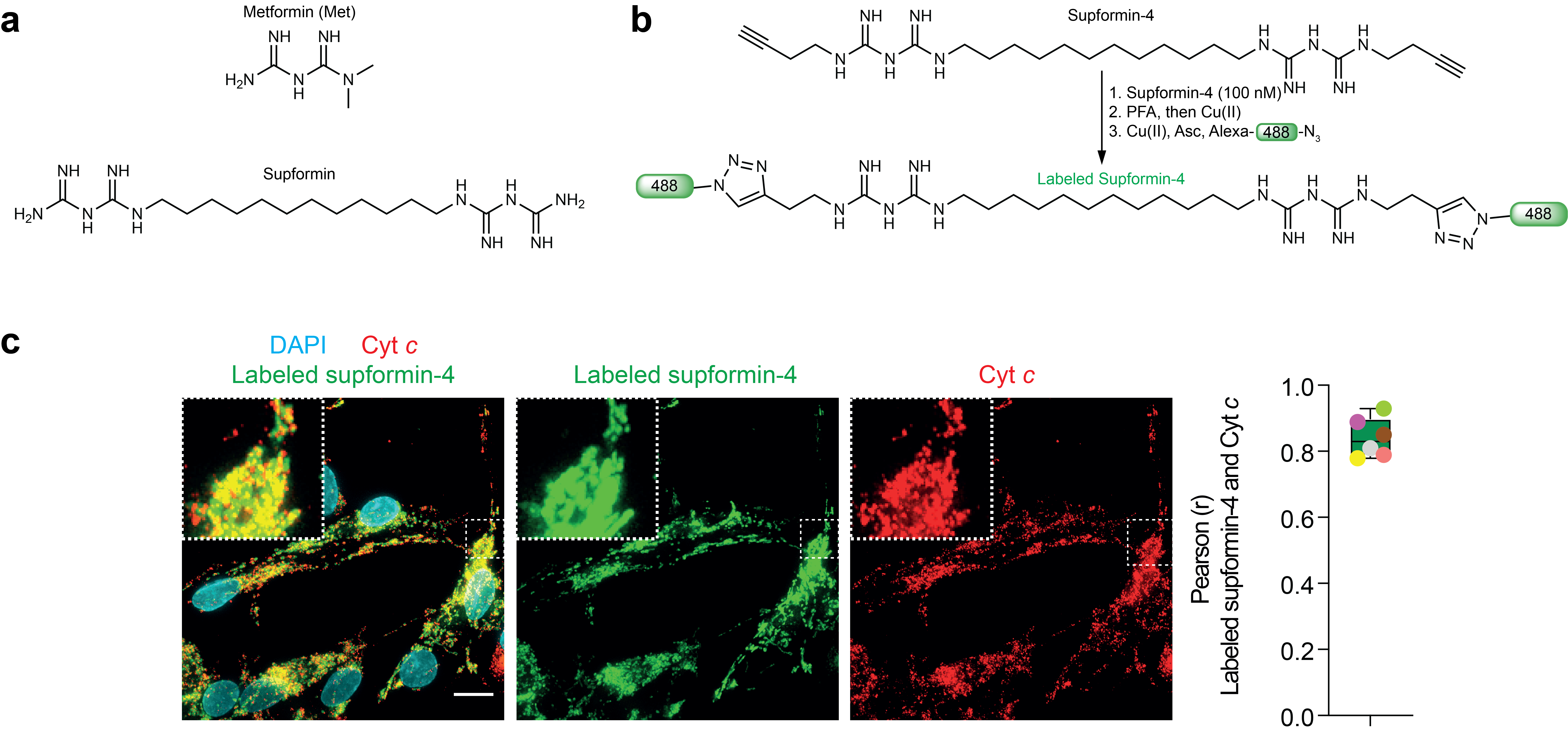
In this study we then evaluated the action of supformin on macrophage activation during inflammation. We found that supformin can reprogram cell metabolism in macrophages and reduce total NAD(H) levels. These metabolites are crucial for many metabolic processes, including the Krebs cycle and we observed altered levels of several metabolites, of which acetyl-CoA and alpha-ketoglutarate are key. These two metabolites are crucial for histone demethylation and acetylation in the cell nucleus, thus, changing their levels lead to changes in the epigenetic landscape of the cell. Changes of the epigenetic landscape are inherent with changes in cell phenotype. Indeed, supformin treatment lead to epigenetic reprogramming into a less inflammatory state.
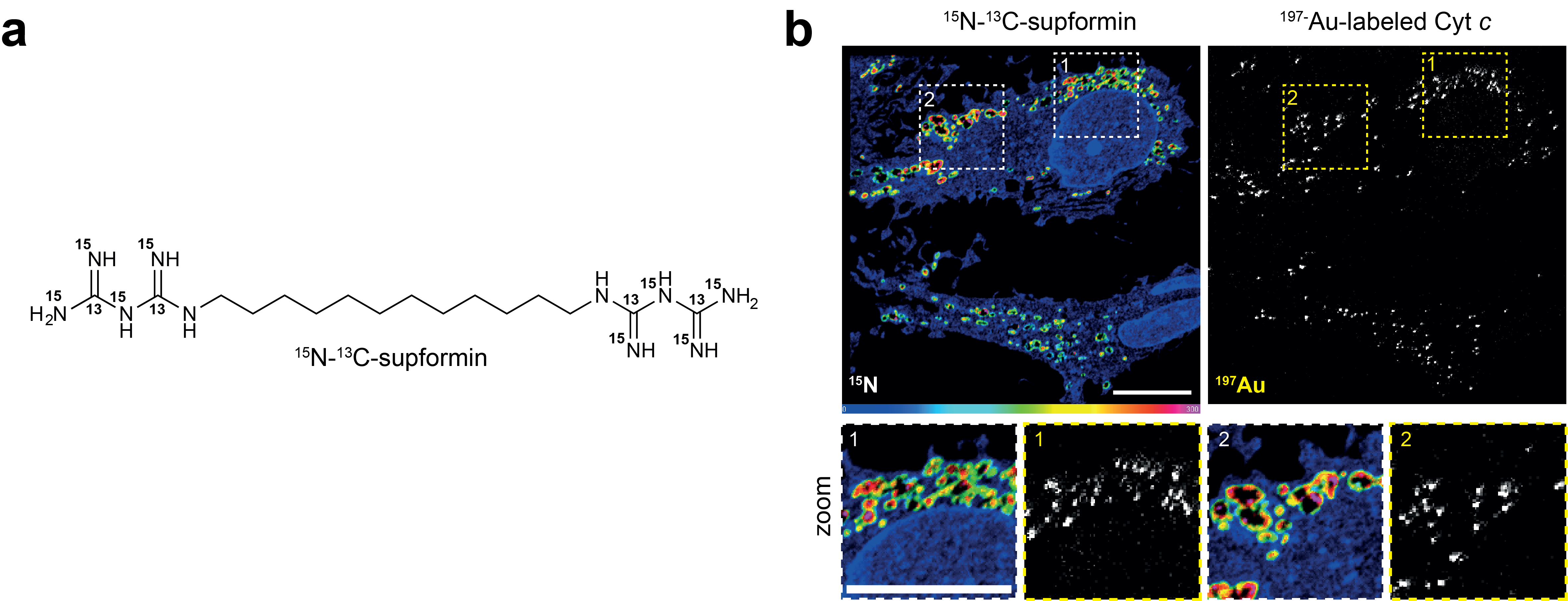
NanoSIMS imaging of isotopologues
In this study we also applied another approach, using an isotopologue of supfomin and imaging this with NanoSIMS (nanoscale secondary ion mass spectrometry). This is essentially a mass spectrometry approach to image distributions in cells. For this, samples are fixed and prepared akin to samples for electron microscopy. Unfortunately, these machines are expensive and only few exist in the world. We are fortunate to have this technology at Institut Curie, who are also open for external collaborations. Here, we used a gold labelled antibody against cyt c to label mitochondria and then imaged gold and a 15N-13C-supformin isotopologue. We previously employed NanoSIMS in 2020 to detect iron increase in mesenchymal cancer cells during epithelail-to-mesenchymal transition5.
References
1 J.B. Leathes, M.B. OXF., F.R.S. The Lancet, THE BIRTH OF CHEMICAL BIOLOGY. doi: 10.1016/S0140-6736(00)77287-4
2 Nat Chem. 2015 Sep;7(9):744-51. doi: 10.1038/nchem.2302
3 Nature Reviews Chemistry volume 2, pages202–215 (2018)
4 Nature. 2023 May;617(7960):386-394. doi: 10.1038/s41586-023-06017-4
5 Nat Chem. 2020 Oct;12(10):929-938. doi: 10.1038/s41557-020-0513-5
6 Nature Chemistry volume 9, pages1025–1033 (2017)
7 Nature, 2025, Activation of lysosomal iron triggers ferroptosis in cancer
Reference: Solier et al., A druggable copper-signalling pathway that drives inflammation, Nature, 2023, 617, 386–394, doi:10.1038/s41586-023-06017-4
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in