Impaired Wnt signaling and the predominantly inattentive ADHD
Published in Neuroscience

Attention-deficit/hyperactivity disorder (ADHD) is the most diagnosed disorder affecting neurobehavioral functions in children, marked by a ubiquitous display of inattention, impulsivity, and hyperactivity, which often persist into adulthood. These symptoms are expressed to different extents, forming individual variations in the balance of symptoms identified and classified by the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) as predominantly inattentive (ADHD-PI), hyperactive-impulsive (ADHD-HI), and combined (ADHD-C) type. Although the exact etiology has remained largely unknown, studies have implicated various gene networks in the development of ADHD. For this reason, identifying domain-specific genetic risk variants would help us uncover the potential genetic mechanism of ADHD, as it may reduce phenotypic heterogeneity and enhance the strengths of genetic studies.
Primarily, children diagnosed with ADHD-PI often struggle with impairments in overall executive function, including temporal processing and visuospatial memory, among others, believed to be subserved by the hippocampal region. The specific genetic or protein factors involved and how they shape the structures of the hippocampal region to influence ADHD behavior remain poorly understood. Besides the recognized multifactorial ADHD etiological factors, current evidence suggests that the interface between genetic and environmental factors, particularly Wnt signaling pathways, might significantly contribute to ADHD. The extracellular Wnt signaling stimulates numerous intracellular signaling cascades involved in various cellular functions, including neural stem cell fate determination, migration and polarity, and neural patterning and organogenesis during embryonic and adult brain development through the transcriptional coactivator catenin beta-1 (β-catenin; Ctnnb1), constituting the Wnt/β-catenin signaling pathway.
As a 'bottom-up' experimental tool, hippocampal dentate gyri (DG) tissues from a mouse model overexpressing thyroid hormone-responsive protein overexpressing (THRSP OE) with defining characteristics of ADHD-PI were utilized in the proteomics study. Also, the effects of environmental enrichment on behavior and gene expression were evaluated, and comparisons were made using wild-type (WT) and Thrsp knockout (THRSP KO) strains.
Our study identified the hippocampal DG molecular signatures in an ADHD-PI mouse model overexpressing the Thrsp gene rather than typical "culprits," such as the dopaminergic or thyroid hormone dysfunction hypothesis in ADHD, also identified in our previous studies (Custodio et al., Neuroscience, 2018; Custodio et al.; Commun Biol, 2021). These findings connect with the ancient evolutionary Wnt signaling pathways critical for cell fate determination, migration, polarity, neural patterning, and organogenesis. This, along with the previous and recent discoveries, supports the role of Wnt signaling in neurological disorders, particularly ADHD. Moreover, the integration of environmental enrichment with treadmill exercise has proven effective in improving not only behavior but also some altered molecular aspects in the hippocampal DG of THRSP OE mice, supporting the benefits conferred by non-pharmacological interventions, such as environmental modifications and exercise in improving the signs and symptoms of ADHD.
Future goals are directed towards identifying THRSP single-nucleotide polymorphisms in humans, particularly in children with ADHD, to further support or verify the utility of Thrsp/THRSP as a potential biomarker for ADHD-PI and to validate the use of THRSP OE mice in modeling this distinct neurobehavioral presentation.
While there is still much to understand, our investigation provides significant findings that could be valuable in advancing ADHD research.
This work was made possible by grants from the Bio & Medical Technology Development Program of the National Research Foundation (NRF) (2016R1D1A1B02010387; 2020M3E5D9080791) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (HI19C0844) of the government of South Korea. The poster image was made using Biorender (https://biorender.com/).
Please read our original scientific article published in Communications Biology to learn more.
Dr. Raly James Perez Custodio, a former Researcher at the Institute for New Drug Development at the College of Pharmacy at Jeonbuk National University (Jeonju) and Uimyung Research Institute for Neuroscience at Sahmyook University (Seoul) in South Korea, wrote this Blog. He recently joined the Ergonomics Department at Leibniz Research Centre for Working Environment and Human Factors – IfADo in Dortmund, Germany, where he continues conducting preclinical behavioral studies in mouse disease models.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026

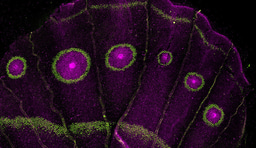
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in