In situ observation of ligand-induced electrocatalytic microenvironment formation at the nanometer scale
Ligand-capped nanoparticles (NPs) find diverse applications in nanoscience, spanning from nanomedicine to optoelectronics and catalysis. Traditionally, the research emphasis lies on the metallic NP core, as it typically dictates the functionality of the overall NP-ligand system, while the behavior of the ligand under corresponding operating conditions and its potential influence on NP functionalities are commonly disregarded. However, an increasing number of research suggests that exploring ligand behavior under operating conditions can yield advantageous effects, and harnessing controllable ligand behavior can result in innovative functionalities. For instance, in drug delivery using nanoparticles, precise dosage control can be achieved through the controllable detachment of the ligand under specific external stimuli, such as pH variations. Therefore, studying the NP-ligand system from the perspective of ligand may be highly rewarding. A better understanding of the ligand-metal interaction under operating conditions introduces an additional dimension for designing NP-ligand systems with diverse and sophisticated functionalities.
In our recent paper published in Nature Catalysis (https://www.nature.com/articles/s41929-024-01119-2), we specifically investigated how the ligand on the NP surface responds to electrochemical conditions and its impact on catalytic performance. We utilized Ag NPs capped by tetradecylphosphonic acid ligands as electrocatalysts, a system invented in our group several years ago, for which we already possess a basic understanding. The system exhibits high activity and selectivity in electrochemically converting CO2 to CO with low overpotential. Our hypothesis posited that the activity originates from a microenvironment established by bias-induced unique ligand behavior. However, understanding how electrochemical bias modulates the interactions between the ligand and Ag surface, eventually leading to the formation of a favorable microenvironment, remains elusive.
To address this question, we sought a technique capable of directly tracking the 'fate of ligands' on NP surfaces under electrochemical conditions. Vibrational spectroscopy, with low excitation energy, emerged as a suitable candidate. It provides a characteristic fingerprint of the ligand while minimizing radiation damage to organic ligands. However, traditional vibrational spectroscopies generally offer spectra information averaged over large areas containing NPs with different geometric features, size/shape, and aggregation statuses. Considering the inherent heterogeneity of NPs and the substantial restructuring of the metallic NP surface, ligands attaching to different positions of a nanoparticle may experience different field strengths, leading to varied behavior. Thus, we required a spectroscopic technique with chemical sensitivity, in situ capability, and spatial resolution on the order of NP size simultaneously. Nanospectroscopy, coupled with a compatible liquid cell, provided the solution (Fig.1).
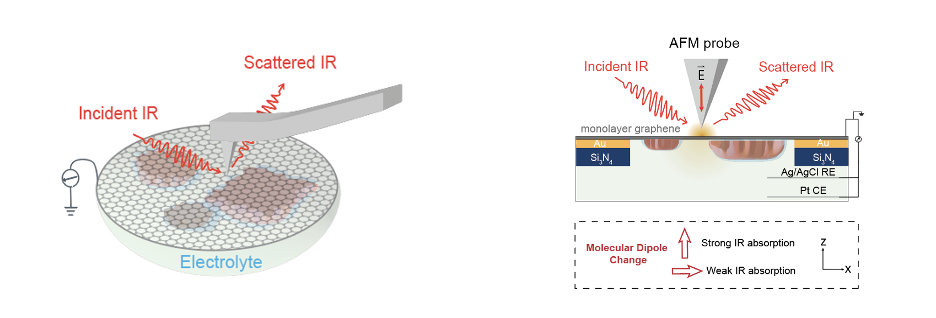
Fig 1. Schematics of the setup of in situ nano-FTIR.
In this study, we employed in situ infrared nanospectroscopy (nano-FTIR) to obtain real-time dynamic molecular information of surface ligands with nanometer spatial precision under electrochemical conditions and discussed its impact on the catalytic performance of the system. Nano-FTIR, in addition to its high spatial resolution, possesses a unique polarization that enables a dramatic change in the intensity of orthogonal vibrations (like νs (P-O) and νas (P-O) of ligands head group) in response to subtle modifications of the binding configuration or orientation of the surface ligand. Characterized by a unique intensity boost in the νas (P-O) mode, nano-FTIR captured the ligand configuration change from the initial bidentate state to the monodentate state on the aggregating nanoparticles. Interestingly, nano-FTIR also captured the bias-modulated orientation change of the monodentate structure. Exploiting the high spatial resolution of nano-FTIR allowed a direct comparison of ligand behavior on NPs with different sizes and evolving statuses, revealing that the bidentate to monodentate transformation occurs universally across all NP surfaces. Importantly, our finding concludes that NP size exerts minimal influence on bias-induced ligand configuration changes, a conclusion impossible to draw using traditional spectroscopy. When the ligand is in a monodentate structure, the remaining covalent interaction between the ligand and Ag surface blocks the accessibility of electrolyte species to the Ag surface, providing no evidence of CO2 reduction under the monodentate configuration.
The current nano-FTIR/graphene cell setup faces fragility issues with the graphene membrane under greater bias, where CO2 reduction occurs. To capture the final ligand state supporting CO2 reduction, we also employed surface-enhanced Raman (SERS). SERS not only provided additional evidence of bidentate to monodentate transformation but also captured the monodentate to free ligand transformation at a more negative potential. Additionally, we observed that once the ligand is transferred to the free ligand, a local pH variation is detected, evidence of the onset of CO2reduction. This consecutive dissociation of ligands, further supported by DFT simulations, offers a real-time picture of how bias modulates ligand-NP interactions to form an electrocatalytic microenvironment. Non-covalent interactions between ligand alkyl chains help maintain a stable shell of detached ligands around the NP surface. Ligand detachment not only provides space for the insertion of cations and reactants but also creates a negatively charged layer that can modulate cations' solvation shell, stabilizing key intermediates at a relatively low overpotential, ultimately leading to high catalytic activity. Therefore, we utilized a suite of in situ techniques and successfully elucidated an electrocatalytic microenvironment formation mechanism triggered by bias-induced variation of the ligand structure.
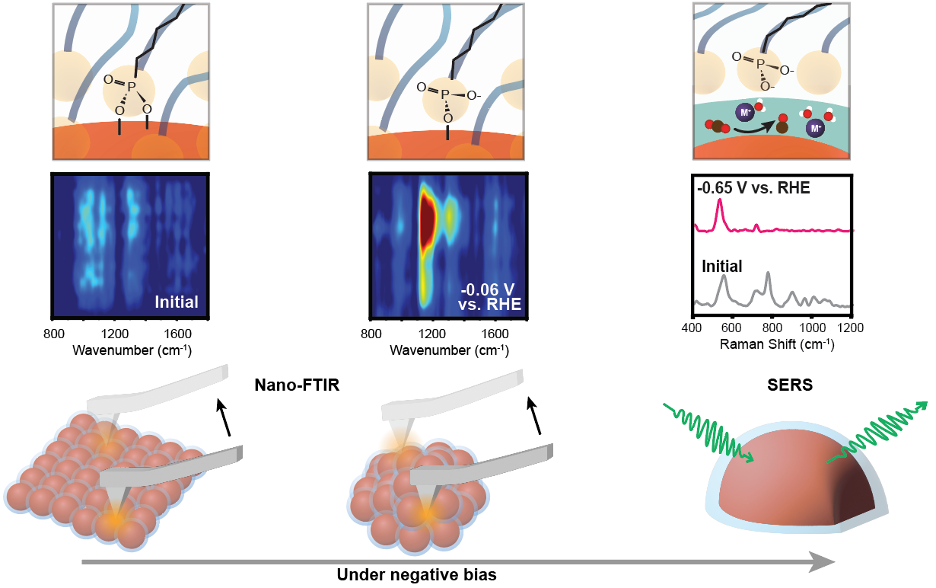
Fig. 2. Real-time molecular picture of microenvironment formation probed by in situ nano-FTIR and SERS
In the end, we emphasize that while the ligand is typically considered a 'bystander' in most NP-ligand system applications, controlled ligand dynamics exhibit significant potential to tune or even determine the functionalities of the NP-ligand system. With the aid of newly developed in situ vibrational spectroscopies with high spatial resolution, a real-time molecular-level understanding of ligand dynamics under relevant operating conditions can be obtained. This is crucial for providing fundamental guiding principles for the rational design of responsive ligand-NP systems with precise dosage-, spatial-, and temporal-control across medicine, optoelectronics
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.
Your space to connect: The Fuel cell technologies Hub
A new Communities’ space to connect, collaborate, and explore research on Electrochemistry, Chemical Engineering, and Fuel Cells!
Continue reading announcement





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in