Inhibition of TGF-β signaling, invasion, and growth of cutaneous squamous cell carcinoma by PLX8394
Published in Cancer

Cutaneous squamous cell carcinoma (cSCC) is the most common metastatic skin cancer with increasing incidence worldwide. At present, in the absence of targeted effective treatment, the prognosis of patients with metastatic disease remains poor. To address this unmet medical need we have searched for novel strategies to block critical signaling pathways in cSCC cells and observed that PLX8394, an orally administrated serine-threonine kinase inhibitor has an optimal substrate spectrum and it obstructs human cSCC progression and invasion in in vitro and in vivo models.
Human cancer cells, including cSCC cells, harbor numerous driver mutations, i.e. mutations that provide a selective growth advantage and promote cancer progression (1, 3). Furthermore, soluble mediators present in tumor microenvironment, such as growth factors and cytokines, may also promote cSCC progression. The large number of driver mutations and the existence of destructive clustered mutations in driver genes raise the question about the potential benefits of optimal wide spectrum inhibitors when compared to highly selective inhibition of a single oncogene or signaling pathway.
In our previous paper we pointed out the interplay between Ras activation and transforming growth factor-β (TGF-β) generated signaling during cSCC invasion (2). Both individual pathways may have important roles in cSCC. Based on a meta-analysis of cSCC driver genes, mutations that activate the MAPK and/or phosphoinositide 3-kinase pathways occurred in 31% of the tumors (3). TGF-β is a multifunctional cytokine that has been considered either as a tumor suppressor or a tumor promoter (4) depending on the cellular context. TGF-β regulates several crucial cellular processes, including proliferation, epithelial-mesenchymal transition (EMT) and differentiation.
In our current paper we show that PLX8394 can block TGF-β signaling in transformed human keratinocytes harboring Ras-mutation, as well as in numerous cSCC cell lines. PLX8394 is a next-generation BRaf inhibitor that does not elicit paradoxical ERK1/2 activation (5), as has been shown to occur with widely used BRaf inhibitors dabrafenib and vemurafenib. PLX8394 has been shown to suppress growth of BRaf mutant lung cancer and melanoma in vivo and it is currently in phase II clinical trial in BRaf mutated cancers (clinicaltrials.gov NCT No: NCT02428712). We demonstrate that in 3D spheroid culture model, PLX8394 blocks TGF-β signaling in transformed keratinocytes and in cSCC cells containing wild-type BRaf. We further show that this inhibitor acts by affecting TGF-β type II receptor kinase activity. Importantly, we do not observe any paradoxical increase in ERK1/2 activation. We also show that by inhibiting TGF-β signaling pathway, PLX8394 attenuates laminin-332 synthesis, thus preventing cancer cell invasion in vitro. The spheroid culture model also shows that PLX8394 is able to simultaneously attenuate TGF-β-activated p38 MAPK pathway, which leads to decreased synthesis of matrix metalloproteinases (MMP), MMP-1 and MMP-13, in cSCC cells. Decreased MMP synthesis in turn prevents the degradation of stromal collagens in cSCC tumors. Finally, we show that PLX8394 is able to inhibit the growth of human cSCC xenografts in vivo and cause large necrotic and keratotic foci inside the PLX8394 tumors. The key findings are collectively presented in the Figure 1.
In accordance with our studies, a positive feedback loop between TGF-β and Ras oncoproteins has recently been proposed by others. During TGF-β induced EMT, alterations in plasma membrane curvature resulted in increased Ras activity, which in turn promoted EMT and resulted in higher invasion potential of cancer cells (6). This cooperation between Ras oncoproteins and TGF-β during EMT could mediate the switch in TGF-β’s role from tumor suppressor to tumor promoter (4), suggesting that dual-targeting of both TGF-β and Ras simultaneously could be more beneficial to cancer therapeutics than targeting them individually.
To conclude, our results indicate that it is possible to find inhibitors for protein serine-threonine kinases with a favorable target spectrum that enables the prevention of cSCC tumor growth. We propose PLX8394 as a promising molecule for future clinical studies in locally advanced and metastatic human cSCC. In general, our results provide a new insight into targeting multiple signaling pathways simultaneously in the battle against cSCC progression and drug resistance.
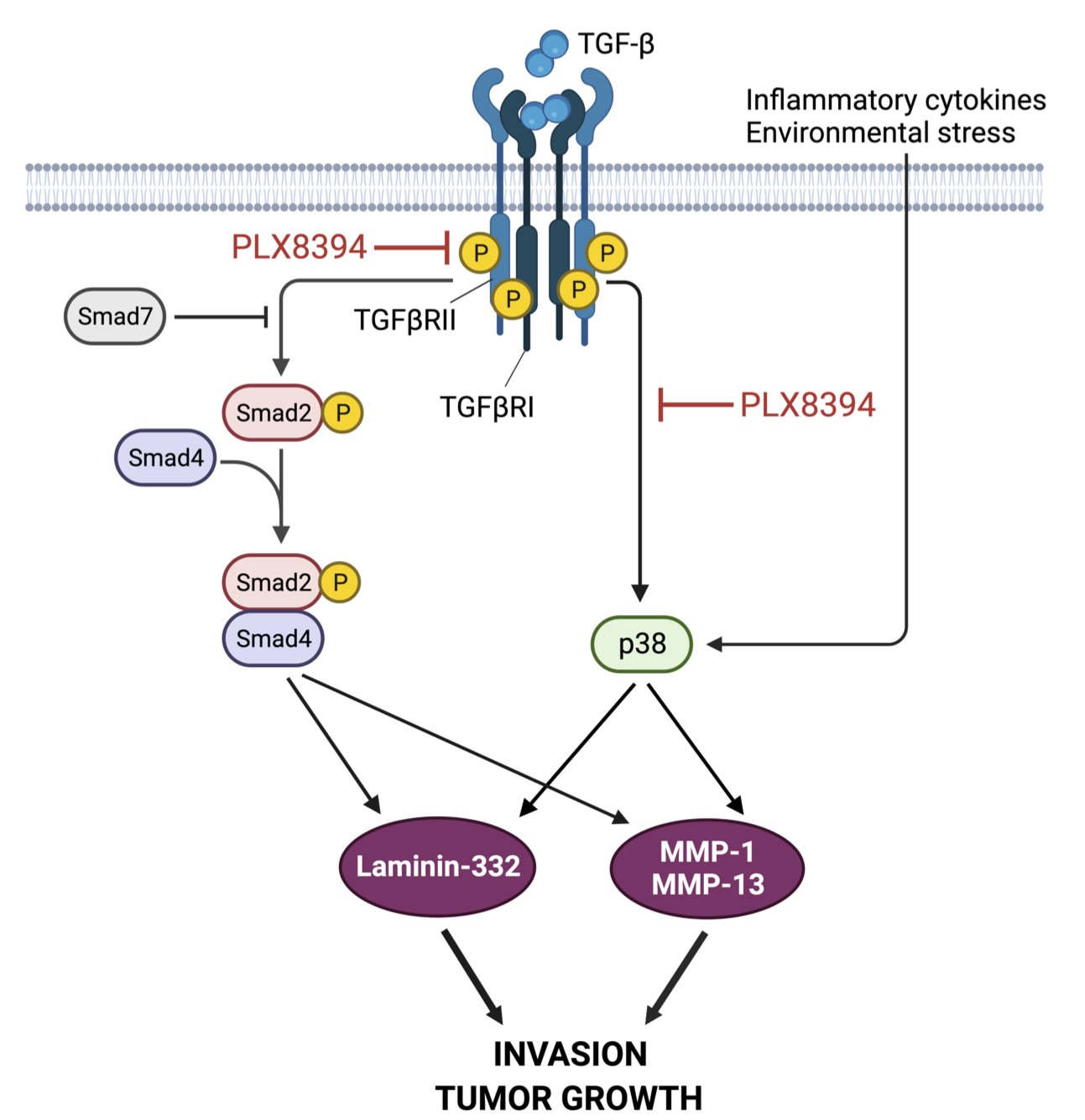
Figure 1. A schematic representation of serine-threonine kinase inhibition by PLX8394. PLX8394 inhibits TGF-β signaling by attenuating phosphorylation of TGFβRII and Smad2. Concurrently, PLX8394 inhibits TGF-β-induced Smad2 and p38 activation resulting in decreased MMP-1 and MMP-13 production. PLX8394 does not inhibit p38 activation induced by inflammatory cytokines or environmental stress. As a result, laminin-332 expression is downregulated leading to decreased cSCC cell invasion and tumor growth. The figure was created with BioRender.com.
References:
- Martínez-Jiménez et al., A compendium of mutational cancer driver genes. Nat Rev Cancer 20 (10):555-572 (2020).
- Siljamäki et al., H-Ras activation and fibroblast-induced TGF-β signaling promote laminin-332 accumulation and invasion in cutaneous squamous cell carcinoma. Matrix Biol. 87:26-47 (2020).
- Chang & Shain, The landscape of driver mutations in cutaneous squamous cell carcinoma. NPJ genomic Med. 6, 1-10 (2021).
- Kuburich et al., Proactive and reactive roles of TGF-β in cancer. Semin Cancer Biol. 95:120-139 (2023).
- Zhang et al., RAF inhibitors that evade paradoxical MAPK pathway activation. Nature 526, 583-586 (2015).
- Damalas et al., TGFβ-induced changes in membrane curvature influence Ras oncoprotein membrane localization. Sci Rep. 5; 2(1):13486 (2022).
Follow the Topic
-
Oncogene

This journal aims to make substantial advances in our knowledge of processes that contribute to cancer by publishing outstanding research.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in