Integrating case reports into systematic reviews
Published in Biomedical Research

We were facing a problem: Research courses usually begin by guiding students to search for ideas and problems upon which to build their research projects. This is not an easy matter, especially when the student-researcher’s options are limited to systematic reviews and meta-analyses.
Because of the intense global competition, and the power of these research methodologies and their relative ease of application (logistically and legally), finding a novel, innovative idea on which to conduct a study becomes extremely difficult—especially for newcomers to the world of medical scientific research.
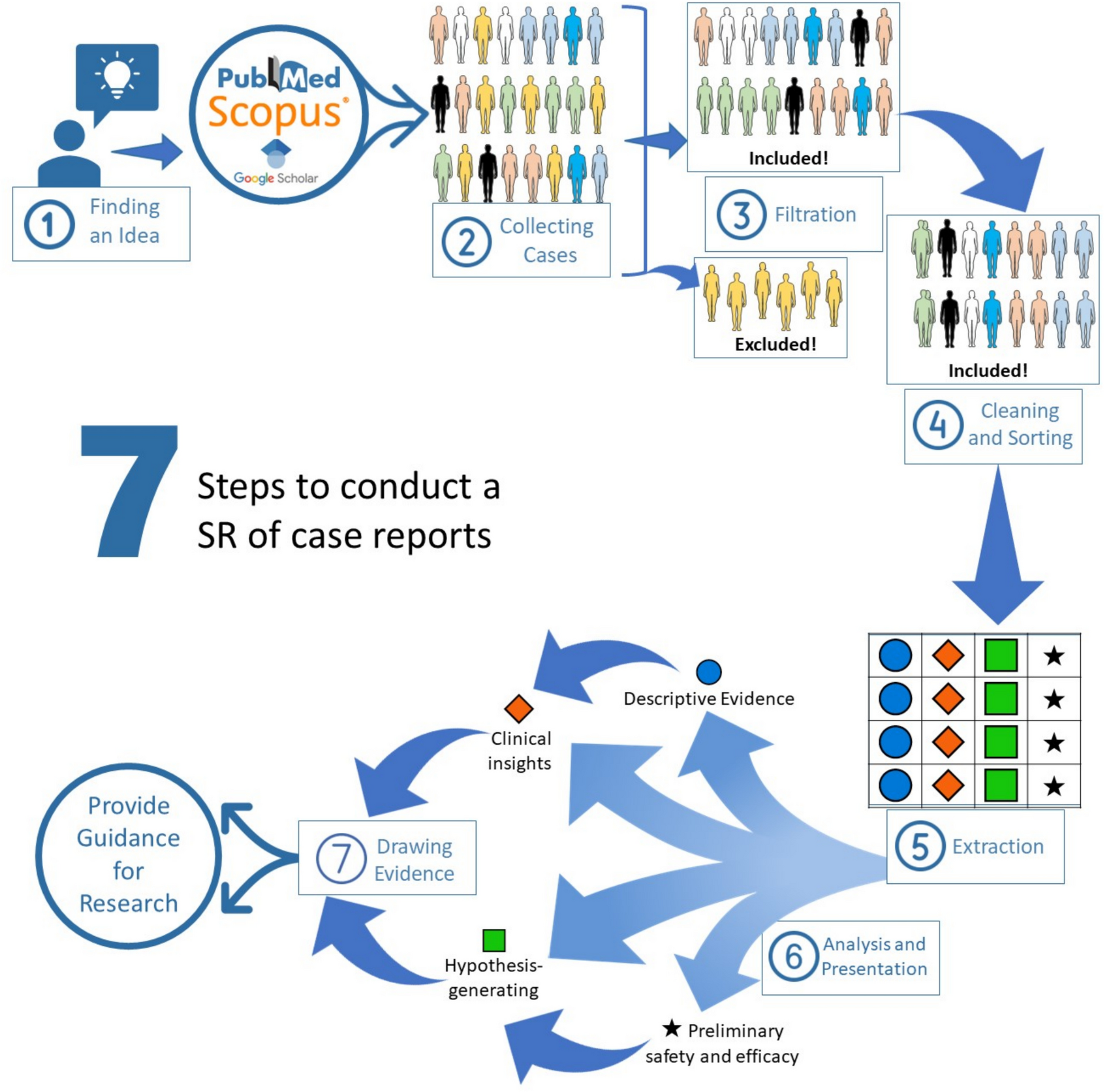
Taha and I began thinking about solutions and reviewing all research methodologies until we were struck by this thought: systematic reviews are conducted only on studies of specific levels, each review collecting a set of studies that describe a large number of patients (the study sample).
But this approach carries an essential problem: for the researcher to be able to conduct a systematic review, there needs to exist a huge number of studies that have progressed up the evidence-based medicine hierarchy, reaching at least clinical trials and cohort studies. What about rare diseases, which have only a few case reports?
Here came the idea — a systematic review of case reports! We began reading and researching, and after several days, we came up with the first formulation of a research methodology that allows for conducting such a “systematic review of case reports” addressing a major problem faced by many new students and researchers, or those who lack access to direct data and primary studies.
Today, the paper explaining this methodology has been published, bearing the signature of Taha & Abuawwad, after four years of continuous research work.
Follow the Topic
-
Systematic Reviews

This journal encompasses all aspects of the design, conduct and reporting of systematic reviews, including protocols, reviews related to a very broad definition of health, rapid reviews, updates of already completed reviews, and methods research related to the science of systematic reviews.
Related Collections
With Collections, you can get published faster and increase your visibility.
Realist Synthesis
Systematic Reviews calls for submissions to our new collection on Realist Synthesis.
This collection invites realist reviews that explore complex interventions and systems across sectors, showcasing the versatility and depth of realist methodology. While realist synthesis has traditionally been rooted in health research, this collection aims to broaden its reach by highlighting work in international development, conservation, climate change, social services, social innovation, and other areas within the social sciences. Submissions from the health sector will, however, still be considered.
We are particularly interested in contributions that demonstrate how realist approaches can inform policy, practice, and theory in diverse contexts. In addition, we strongly encourage submissions from early career researchers, whose innovative perspectives and methodological rigor are vital to the continued evolution of realist review practice.
By bringing together a wide range of realist syntheses, this collection seeks to foster interdisciplinary dialogue and promote the use of realist methods in addressing complex societal challenges.
All submissions in this collection undergo the journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Aug 18, 2026
Automation in systematic reviews
Systematic Reviews invites you to submit to our new thematic series, ‘Automation in systematic reviews’.
Systematic reviews have become the foundation of evidence-based practice. Conducting a systematic review is largely a manual process that requires much expertise, time and money. Major advances have been made in the past two decades to improve the methodologies so that systematic reviews are more reliable, though these rigorous new standards add to the time and costs needed to produce high quality reviews. As the demands for systematic reviews increase, there is a need to lower the costs and reduce the time needed to produce them. Until recently, little has been done to improve the efficiency of the systematic review process and to use the computer in innovative ways to make systematic reviews more efficient to produce and useful.
Computers have been used routinely in various steps of a systematic review, such as searching the literature, collecting data with a spreadsheet, maintaining list of studies in a database program, and drafting the reports with a word processor. However, such uses have not taken advantage of advances in new computer technologies that could automate large parts of the systematic review process to significantly improve the efficiency of the process, and the quality and usefulness of the systematic reviews.
In this series, the Editors invite authors to submit articles about innovative uses of computer technologies in producing systematic reviews. These could include, but are not limited to, discussions on the tools and sciences to automate various aspects of the systematic review processes. We are also interested in papers on making systematic reviews computable.
Manuscripts should be formatted according to our submission guidelines and submitted via the online submission system. In the submission system please make sure the correct collection title is chosen on the additional information tab. Please also indicate clearly in the covering letter that the manuscript is to be considered for the ‘Automation in systematic reviews’ series.
For further information, please use the contact us email on the journal website.
Publishing Model: Open Access
Deadline: Ongoing





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in