International multicenter validation of AI-driven ultrasound detection of ovarian cancer
Published in Computational Sciences and General & Internal Medicine
Ovarian Cancer: Diagnostic Challenges and Current Practices
Ovarian cancer is the most lethal gynecologic malignancy, with a five-year survival rate below 50%, primarily due to frequent late-stage diagnoses.1 Benign adnexal masses, common across all age groups, are often asymptomatic and typically detected incidentally during imaging for other purposes.2 Nearly 10% of asymptomatic postmenopausal women have an ovarian lesion, though only 1% are malignant.3 Managing these patients presents significant diagnostic challenges, requiring accurate triage to balance the risks of missing malignancies against unnecessary surgeries.
Conservative management of benign lesions through ultrasound follow-up or, if symptomatic, minimally invasive surgery, reduces morbidity, preserves fertility, and avoids unnecessary healthcare costs.4,5 In contrast, women with suspected ovarian cancer benefit from referral to gynecologic oncology centers, where specialized surgical management increases the likelihood of complete tumor removal, improving survival outcomes.6,7,8
Transvaginal ultrasound is the primary technique used to distinguish benign from malignant ovarian lesions due to its accessibility and high diagnostic accuracy, especially when performed by specialist examiners.9,10 However, a shortage of experienced examiners, particularly in underserved areas, limits access to timely and accurate diagnoses, leading to delays in treatment and increased risks of unnecessary surgical interventions.11,12
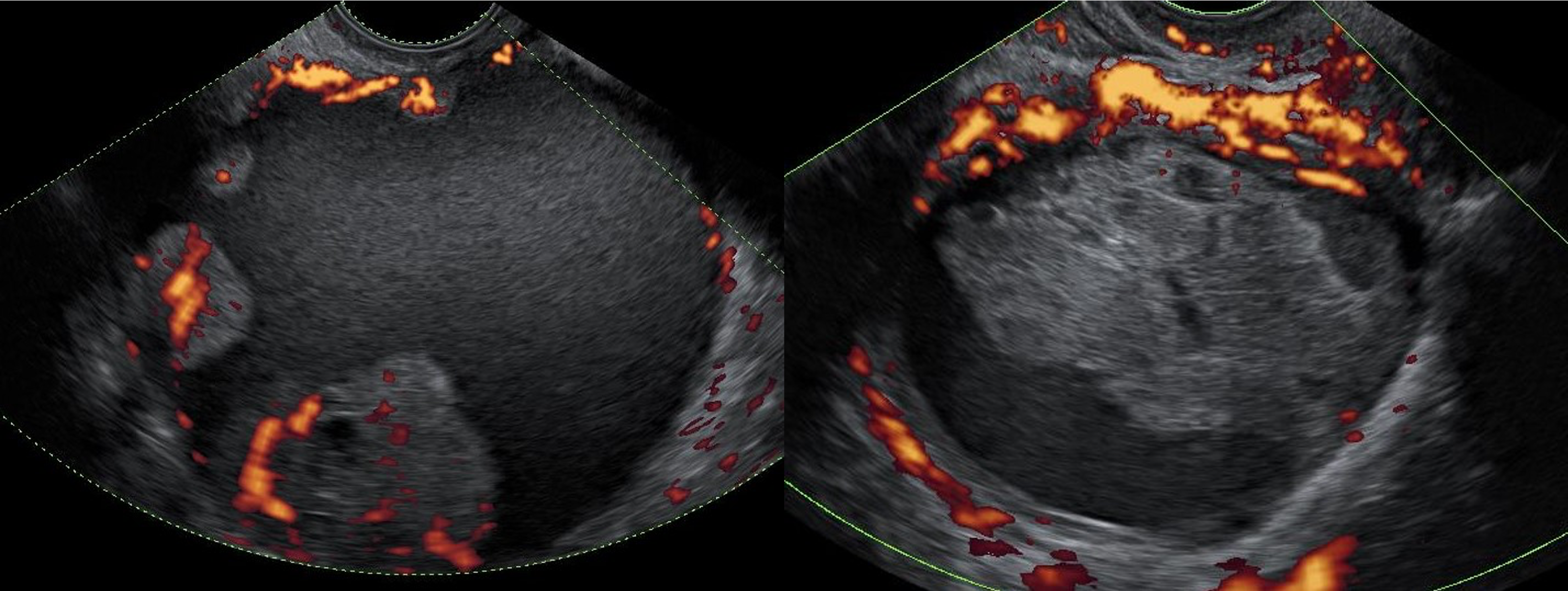
Ultrasound images of two ovarian lesions—an ovarian cancer (left) and a benign functional lesion (right)—with power Doppler visualizing their vascular activity.
Advancing Ultrasound Diagnostics with AI Support
The increasing shortage of specialist ultrasound examiners has created significant challenges in the timely and accurate diagnosis of ovarian lesions, resulting in treatment delays and unnecessary surgeries. This highlights the need for innovative approaches to support medical professionals in delivering high-quality care. Our research team, based at the Karolinska Institute and the Science for Life Laboratory (SciLifeLab), in collaboration with KTH Royal Institute of Technology, the Stockholm South General Hospital (Södersjukhuset), and Intelligyn—a company specializing in AI-driven diagnostic tools—is exploring the potential of artificial intelligence (AI) in enhancing the diagnosis of ovarian tumors.

Recent advancements in AI, particularly deep learning, have shown remarkable promise in medical imaging diagnostics, achieving performance comparable to medical specialists in areas such as dermatology and breast cancer screening.13,14 Building on this progress, our earlier work demonstrated that deep learning models can accurately distinguish between benign and malignant ovarian tumors, matching the diagnostic accuracy of experienced ultrasound examiners.15
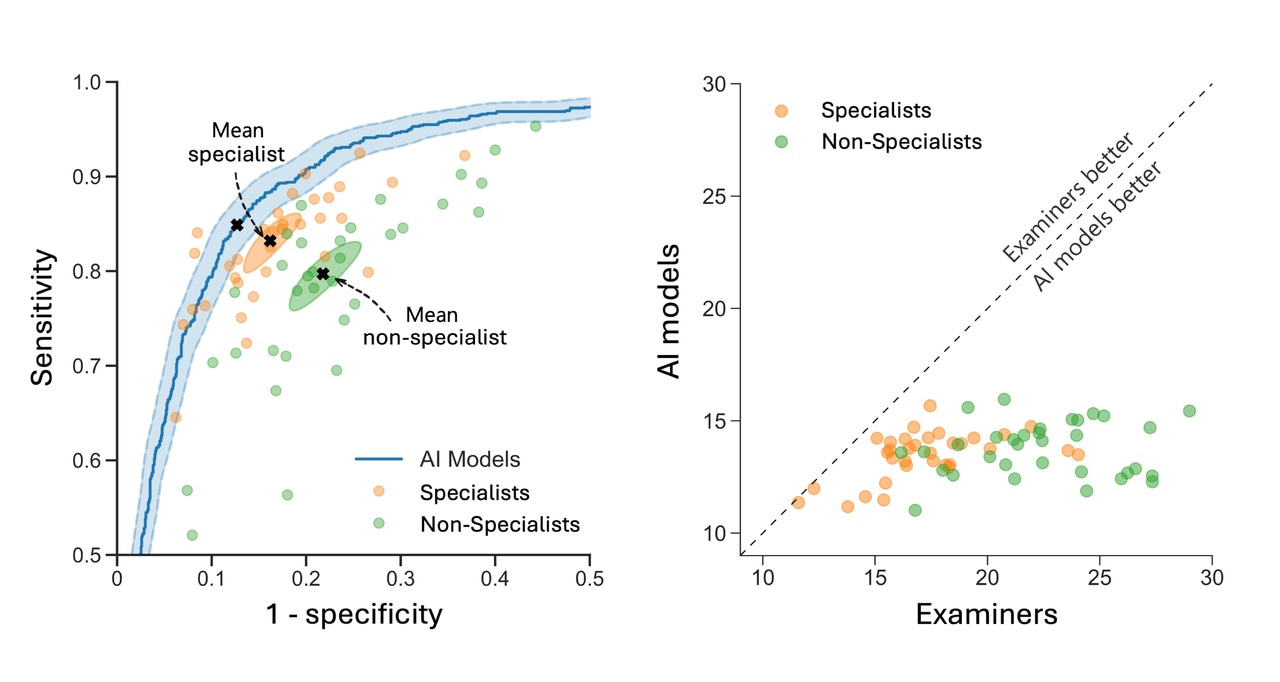
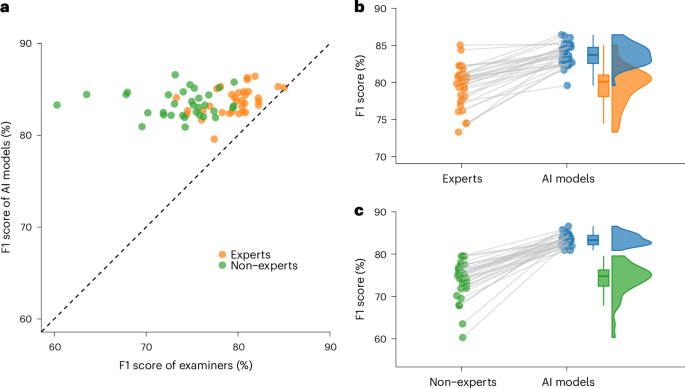
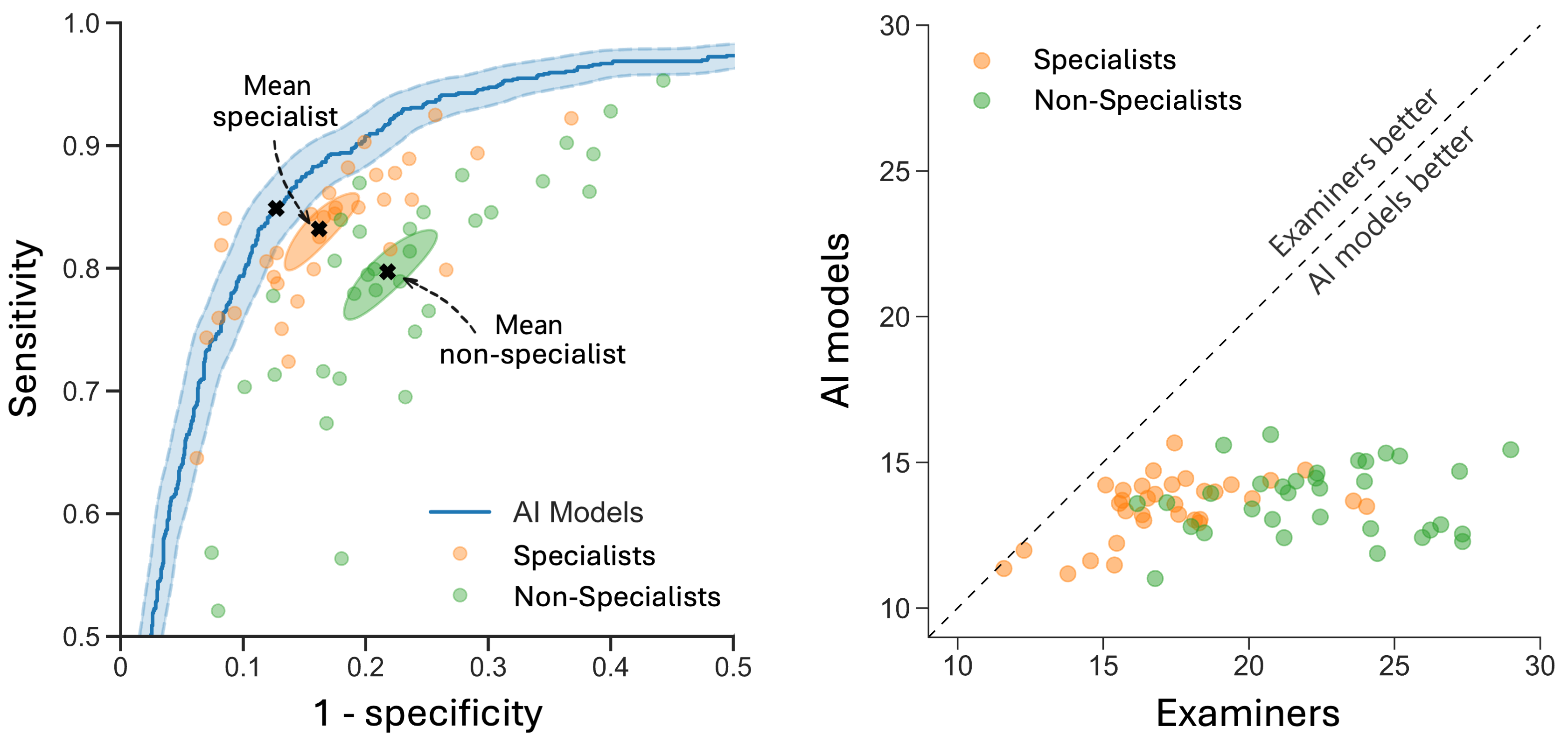
In a recent large-scale international validation study in Nature Medicine, we validated these findings across diverse populations and ultrasound equipment.16 This study included 3,652 patients from 20 centers in 8 countries and the AI model consistently outperformed 66 ultrasound examiners, including 33 specialists with a median of 17 years of experience. Additionally, a retrospective simulation demonstrated the potential of the AI model to serve as a second independent reader, supporting non-specialist examiners in a clinical triage workflow. In this setup, specialists reviewed only cases of disagreement, leading to a 63% reduction in referrals to specialists and an 18% reduction in incorrect diagnoses. These findings highlight the potential of diagnostic AI support to significantly enhance efficiency and optimize the use of limited healthcare resources.
Comparison of AI models and human examiners. The left figure shows the ROC curve for the AI models (blue) alongside human examiners, with specialists (orange) and non-specialists (green) as individual points. The right figure compares the error rates of the AI models and individual examiners on the same patient sets, where points below the dashed diagonal indicate AI outperforming human examiners.
Ethical Approvals
This study obtained necessary ethical approvals from the Swedish Ethics Review Authority (Etikprövningsmyndigheten) (Dnr 2020-06919).
Other Ongoing and Planned Studies
Several other studies are underway or planned to further explore the impact of AI in ovarian tumor diagnostics:
- Retrospective Multi-Reader, Multi-Case (MRMC) Study: This study will involve approximately 30 examiners with different levels of expertise assessing at least 500 ovarian lesions twice, once with and once without AI support, to evaluate the impact of AI support on diagnostic accuracy and inter-observer variability.
- Prospective Multicenter Study (OV-AID Phase I): This ongoing study, which began in February 2021, compares the AI model stand-alone with the clinical assessment of examining physicians, with examiners blinded to the AI predictions. Read more here: ISRCTN88222986.
- Clinical Feasibility Study (IntelligynAI-FS): This clinical study focuses on the practical integration of AI support into clinical workflows, examining its effect on diagnostic confidence and examiner decision-making. Read more here: ISRCTN90989270.
- Randomized Controlled Multicenter Study (OV-AID Phase II): Planned to start in 2026, this study will explore the effect of AI support on patient outcomes, management, and cost-benefit measures in ultrasound assessment of ovarian lesions.
Join Us on Our Journey
For those interested in learning more about this study and staying updated on our progress, we encourage you to read the full article and follow Intelligyn's website and our forthcoming publications. Together, we can move closer to ensuring that innovative AI solutions meet the needs of both patients and healthcare providers.
Authors
Filip Christiansen*, Emir Konuk*, Adithya Raju Ganesham, Robert Welch, Joana Palés Huix, Artur Czekierdowski, Francesco Paolo Giuseppe Leone, Lucia Anna Haak, Robert Fruscio, Adrius Gaurilcikas, Dorella Franchi, Daniela Fischerova, Elisa Mor, Luca Savelli, Maria Àngela Pascual, Marek Jerzy Kudla, Stefano Guerriero, Francesca Buonomo, Karina Liuba, Nina Montik, Juan Luis Alcázar, Ekaterini Domali, Nelinda Catherine P. Pangilinan, Chiara Carella, Maria Munaretto, Petra Saskova, Debora Verri, Chiara Visenzi, Pawel Herman, Kevin Smith†, and Elisabeth Epstein†.
* Joint first authors, † Joint last authors
Author Affiliations
Acknowledgments
This study is made possible through the support of the Swedish Research Council (Vetenskapsrådet), the Swedish Cancer Society (Cancerfonden), the Stockholm Regional Council (Region Stockholm), the Cancer Research Funds of Radiumhemmet (Radiumhemmets Forskningsfonder), and the Wallenberg AI, Autonomous Systems and Software Program (WASP).
We are grateful to all the physicians who participated in the external case review: Juan Luis Alcázar, Bartłomiej Barczyński, Dominika Bednářová, Ida Belfrage, Emelie Bessfelt, Emma Björn, Sonia Bove, Francesca Buonomo, Sofia Bussolaro, Chiara Carella, Artur Czekierdowski, Giovanni Delli Carpini, Ekaterini Domali, Paolo Donarini, Sara Doroldi, Oľga Dubová, Elisabeth Epstein, Dorella Franchi, Filip Frühauf, Robert Fruscio, Chris April H. Garcia, Giorgia Garganese, Adrius Gaurilcikas, Dovile Gaurilcikiene, Migle Gedgaudaite, Roberta Marie Gentile, Francesco Paolo Giuseppe Leone, Stefano Guerriero, Lucia Anna Haak, Jitka Klikarová, Roman Kocián, Klara Krantz Andersson, Emmeline Krook, Marek Jerzy Kudla, Karina Liuba, Agne Lukosiene, Francesco Mezzapesa, Zuzana Michalcová, Anna Minelli, Nina Montik, Elisa Mor, Maria Munaretto, Cristiana Paniga, Maria Àngela Pascual,Ida Pino, Vilma Ravelli, Camilla Robertsson Grossmann, Lottie Säker, Petra Saskova, Carolina Maria Sassu, Luca Savelli, Luisa Scalvi, Linnea Skogvard, Erica Smedberg, Mateusz Stolecki, Michał Szpringer, Noemi Tiszlavicz, Beatriz Valero, Julio Vara García, Debora Verri, Chiara Visenzi, Panagiotis Vlastarakos, Roberto Zanini, and Bettina Zsikai.
We thank Emma Bernell, Rasmus Green, Sanaa Jamil and Stella Wickström for their contribution to the image annotation.
References
- Torre, L. A. et al. Ovarian cancer statistics. CA Cancer J Clin, 68:284–296 (2018). DOI: 10.3322/caac.21456.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 174: evaluation and management of adnexal masses. Obstet Gynecol, 128:e210–e226 (2016). DOI: 10.1097/aog.0000000000001768.
- Sharma, A. et al. Risk of epithelial ovarian cancer in asymptomatic women with ultrasound‐detected ovarian masses: a prospective cohort study within the UK collaborative trial of ovarian cancer screening (UKCTOCS). Ultrasound Obstet Gynecol, 40:338–344 (2012). DOI: 10.1002/uog.12270.
- Yazbek, J. et al. Effect of quality of gynaecological ultrasonography on management of patients with suspected ovarian cancer: a randomised controlled trial. Lancet Oncol, 9:124–131 (2008). DOI:10.1016/s1470-2045(08)70005-6.
- Froyman, W. et al. Risk of complications in patients with conservatively managed ovarian tumours (IOTA5): a 2-year interim analysis of a multicentre, prospective, cohort study. Lancet Oncol, 20:448–458 (2019). DOI: 10.1016/s1470-2045(18)30837-4.
- Vernooij, F. et al. Specialized care and survival of ovarian cancer patients in the Netherlands: nationwide cohort study. J Natl Cancer Inst, 100:399–406 (2008). DOI: 10.1093/jnci/djn033.
- Vergote, I. et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet, 357:176–182 (2001). DOI: 10.1016/s0140-6736(00)03590-x.
- Bristow, R. E. et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol, 41:4065–4076 (2023). DOI: 10.1200/jco.22.02765.
- Timmerman, D. et al. ESGO/ISUOG/IOTA/ESGE Consensus Statement on pre-operative diagnosis of ovarian tumors. Int J Gynecol Cancer, 31:961–982 (2021). DOI: 10.1136/ijgc-2021-002565.
- Van Holsbeke, C. et al. Ultrasound methods to distinguish between malignant and benign adnexal masses in the hands of examiners with different levels of experience. Ultrasound Obstet Gynecol,34:454–461 (2009). DOI: 10.1002/uog.6443.
- Van Holsbeke, C. et al. Ultrasound experience substantially impacts on diagnostic performance and confidence when adnexal masses are classified using pattern recognition. Gynecol Obstet Invest,69:160–168 (2010). DOI: 10.1159/000265012.
- Timmerman, D. et al. Subjective assessment of adnexal masses with the use of ultrasonography: an analysis of interobserver variability and experience. Ultrasound Obstet Gynecol, 13:11–16 (1999). DOI: 10.1046/j.1469-0705.1999.13010011.x.
- Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature, 542:115–118 (2017). DOI: 10.1038/nature21056.
- McKinney, S.M. et al. International evaluation of an AI system for breast cancer screening. Nature,577:89–94 (2020). DOI: 10.1038/s41586-019-1799-6.
- Christiansen, F. et al. Ultrasound image analysis using deep neural networks for discriminating between benign and malignant ovarian tumors: comparison with expert subjective assessment. Ultrasound Obstet Gynecol, 57:155–163 (2021). DOI: 10.1002/uog.23530.
- Christiansen, F. et al. International multicenter validation of AI-driven ultrasound detection of ovarian cancer. Nat Med, 31:189–196 (2025). DOI: 10.1038/s41591-024-03329-4.
Follow the Topic
-
Nature Medicine

This journal encompasses original research ranging from new concepts in human biology and disease pathogenesis to new therapeutic modalities and drug development, to all phases of clinical work, as well as innovative technologies aimed at improving human health.
Related Collections
With Collections, you can get published faster and increase your visibility.
Stem cell-derived therapies
Publishing Model: Hybrid
Deadline: Mar 26, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in