
Explore the Research

Transcriptomics and the origin of obligate parthenogenesis - Heredity
Heredity - Transcriptomics and the origin of obligate parthenogenesis
Over the past few years the Xu lab has dedicated its efforts to understanding the genes and mechanisms involved in the emergence of obligate parthenogenesis. This phenomenon has long captivated evolutionary biologists as it has occurred independently in different multicellular organisms, often facilitated by interspecific hybridization (Neiman et al. 2014). While the diverse cytogenetic manifestations associated with this transition have been well-documented, the precise molecular mechanisms and genes responsible for these cytogenetic changes remain elusive.
In the Xu lab we used the model organism Daphnia, a microcrustacean commonly found in freshwater habitats around North America, to study the emergence of obligate parthenogenesis (OP). The unique reproductive characteristics of Daphnia make it an ideal system for our study. Normally Daphnia reproduce via cyclical parthenogenesis (CP). During favorable environmental conditions mature females reproduce asexually by forming diploid eggs via a modified meiosis, where meiosis I arrests before anaphase I, resulting in no segregation of homologous chromosomes. Meiosis II proceeds normally, leading to the production of diploid subitaneous eggs (Hiruta et al. 2010). Once the environment deteriorates haploid eggs are produced via conventional meiosis, forming resting embryos upon fertilization (Figure 1A). Interestingly, through complex hybridization and introgression events between two sister species D. pulex and D. pulicaria, isolates have emerged that have completely abandoned sexual reproduction and produce both subitaneous eggs and resting eggs via an abortive meiotic process (Figure 1 B, Xu et al. 2013, 2015).
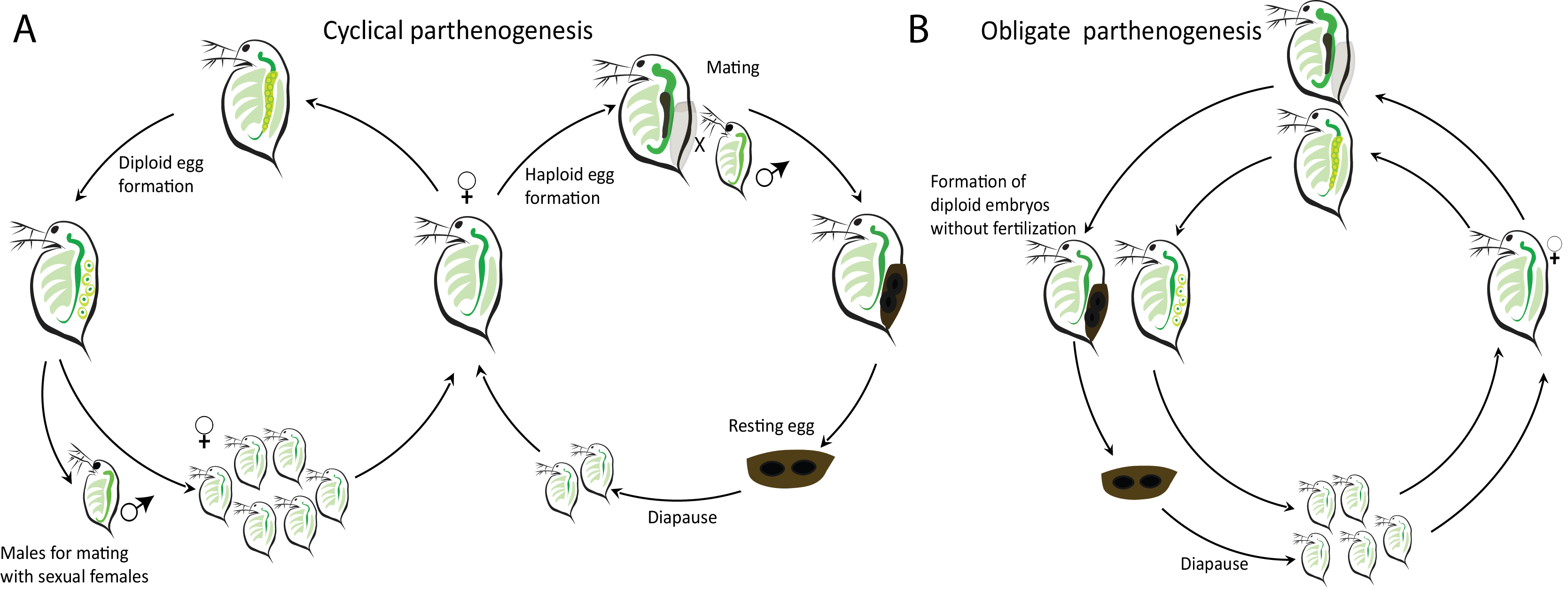
Figure 1. (A) Cyclically parthenogenetic and (B) obligately parthenogenetic life cycles in Daphnia.
This study has been conducted as part of a series with the ultimate goal of understanding the genetic and mechanistic modifications required for OP Daphnia to emerge. Xu et al. 2022 examined the genome-wide expression patterns between OP D. pulex and parental CP D. pulex and D. pulicaria isolates during early resting egg production (Xu et al. 2022), while Huynh et al. 2021 compared gene expression patters between early subitaneous egg and early resting egg production within CP D. pulex and D. pulicaria individuals (Figure 2, Huynh et al. 2021). The results from these two studies strongly suggest that parthenogenesis in both OP (resting egg production) and CP (subitaneous egg production) isolates are associated with a downregulation of meiosis and cell cycle genes and an upregulation of metabolic and biosynthesis genes.

Figure 2. (A) Early subitaneous egg and (B) early resting egg production.
We further expanded on this work by investigating whether OP D. pulex isolates utilize the existing ameiotic germline cell division pathway used in the production of subitaneous eggs for the production of resting eggs. To uncover the cytological modifications in modified meiosis I, we examined the genome-wide gene expression variation and patterns of alternative splicing between early subitaneous and early resting egg parthenogenesis in OP D. pulex isolates (Figure 2). Our results showed an upregulation of meiosis and cell cycle genes, and a divergent expression pattern of metabolism, biosynthesis and signaling pathways (Figure 3A). Additionally, our study provides important candidate genes mapped to the cell cycle, oocyte meiosis, progesterone-mediated oocyte maturation, and Hedgehog signaling pathways for future investigation. One such gene of particular interest to our lab is CDC20, which plays a vital role in activating the anaphase-promoting complex/cyclosome (APC/C) in addition to regulating the progression from metaphase to anaphase through the destruction of cyclin B1 and securing (Jin et al. 2010). Together the results from this study and the previous highlight the differences between reproductive modes in CP and OP individuals, and that the expression of various metabolic, biosynthesis and signaling pathways play an important role in regulating these reproductive modes (Figure 3B).
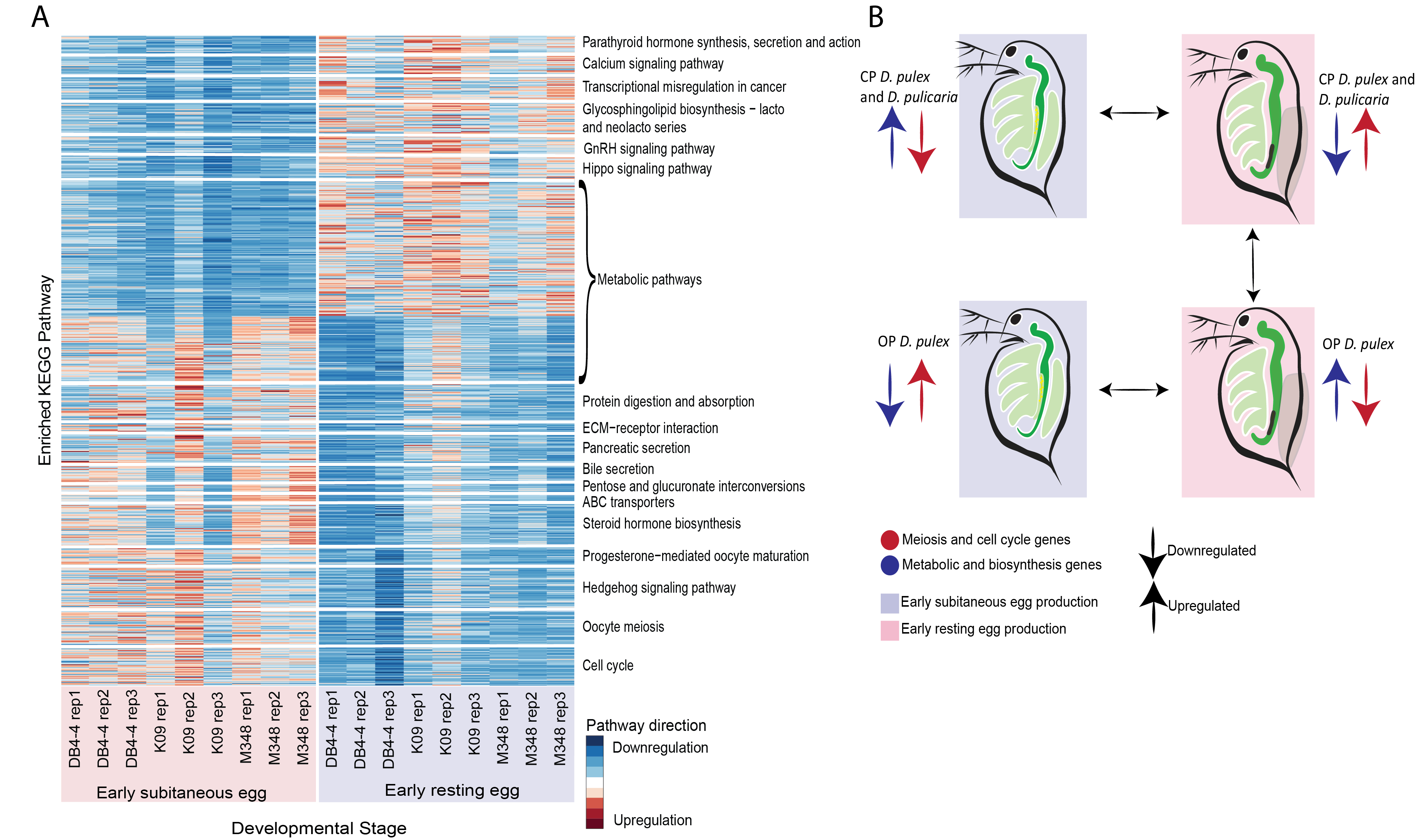
Figure 3. (A) Heatmap illustrating a subset of up- and downregulated KEGG pathways during early subitaneous egg production across all replicates. (B) Summary of pathway regulation across various modes of reproduction in OP and CP Daphnia.
References
1. Hiruta C, Nishida C, Tochinai S (2010) Abortive meiosis in the oogenesis of parthenogenetic Daphnia pulex. Chromosome Res 18(7):833–840
2. Huynh TV, Hall AS, Xu S (2021) The transcriptomic signature of cyclical parthenogenesis (p. 2021.09.27.461985). bioRxiv.
3. Jin F, Hamada M, Malureanu L, Jeganathan KB, Zhou W, Morbeck DE, van Deursen JM (2010) Cdc20 Is Critical for Meiosis I and Fertility of Female Mice. PLOS Genetics, 6(9), e1001147.
4. Neiman M, Sharbel TF, Schwander T (2014) Genetic causes of transitions from sexual reproduction to asexuality in plants and animals. Journal of Evolutionary Biology, 27(7), 1346–1359.
5. Xu S, Innes DJ, Lynch M, Cristescu ME (2013) The role of hybridization in the origin and spread of asexuality in Daphnia. Molecular Ecology, 22(17), 4549–4561.
6. Xu S, Spitze K, Ackerman MS, Ye Z, Bright L, Keith N, Jackson CE, Shaw JR, Lynch M (2015) Hybridization and the Origin of Contagious Asexuality in Daphnia pulex. Molecular Biology and Evolution, 32(12), 3215–3225.
7. Xu S, Huynh TV, Snyman M (2022) The transcriptomic signature of obligate parthenogenesis. Heredity, 128(2), 132–138.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in