Ion Transport on Bubbles
Published in Chemistry

It wasn't meant to happen. My reference electrode was still out of the solution when the working electrode, a nicely cut and polished cube of hematite, was already under tension. I was splitting water. Light-reflecting oxygen bubbles suddenly materialised, defying their inherent buoyancy by remaining stubbornly pinned onto hematite. As I marvelled at the spectacle unravelling through the lens of the video microscope, I wondered what I could do with these bubbles. I had the perfect tool: a Pt ultramicroelectrode (UME), initially intended to map the electrochemical landscape of the hematite surface. Using a piezo stage, I navigated the UME over a bubble, then lowered it to establish contact.
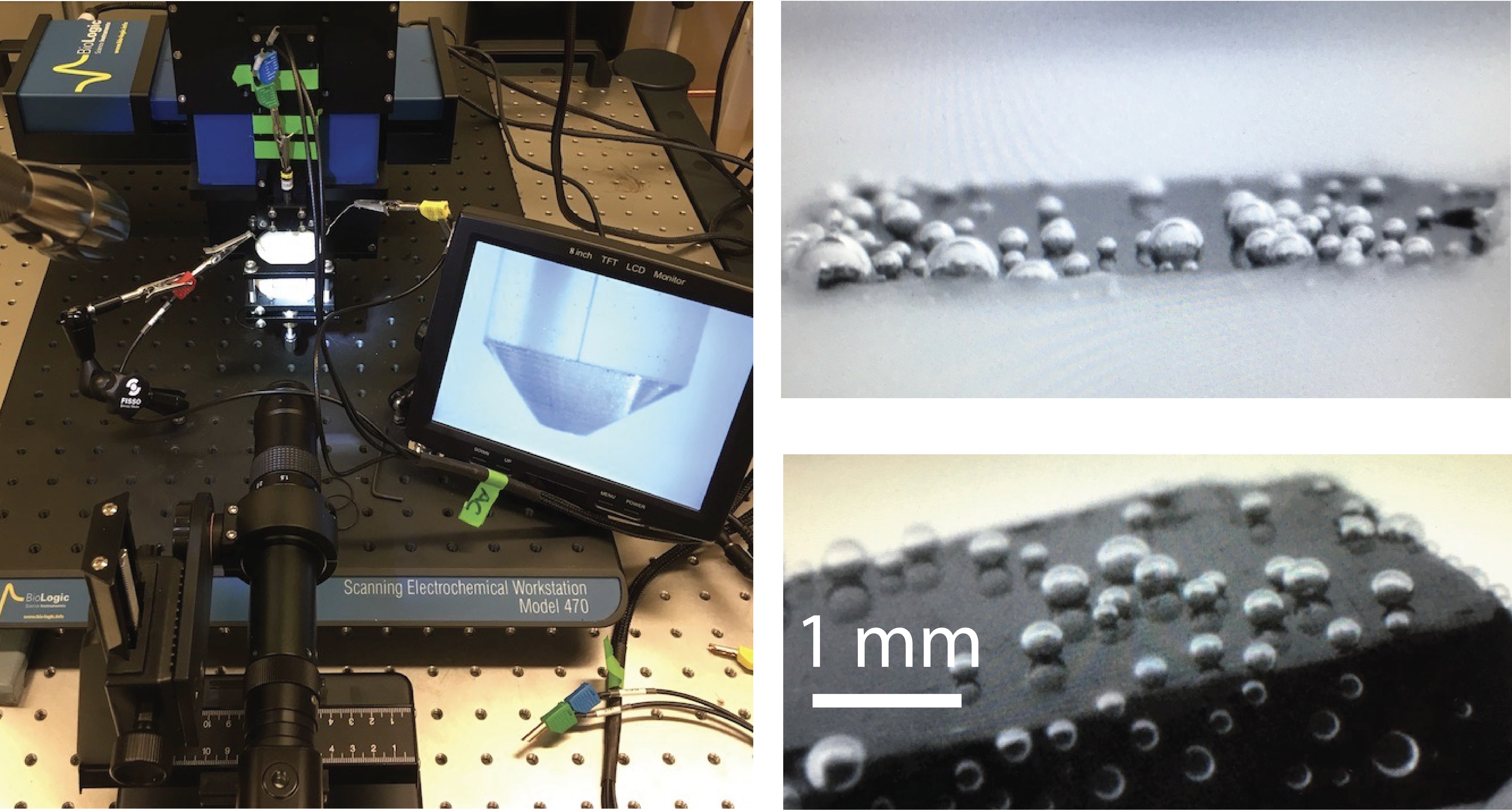
Using the UME, I measured the electrical impedance of the bubble surface in an electrochemical impedance spectroscopy (EIS) experiment. By measuring the impedance while applying an alternating current over a range of frequencies, I found an electrochemical signature akin to a simple resistor-capacitor circuit. This signature reflects ion transport phenomena along the outer skin of gas bubbles.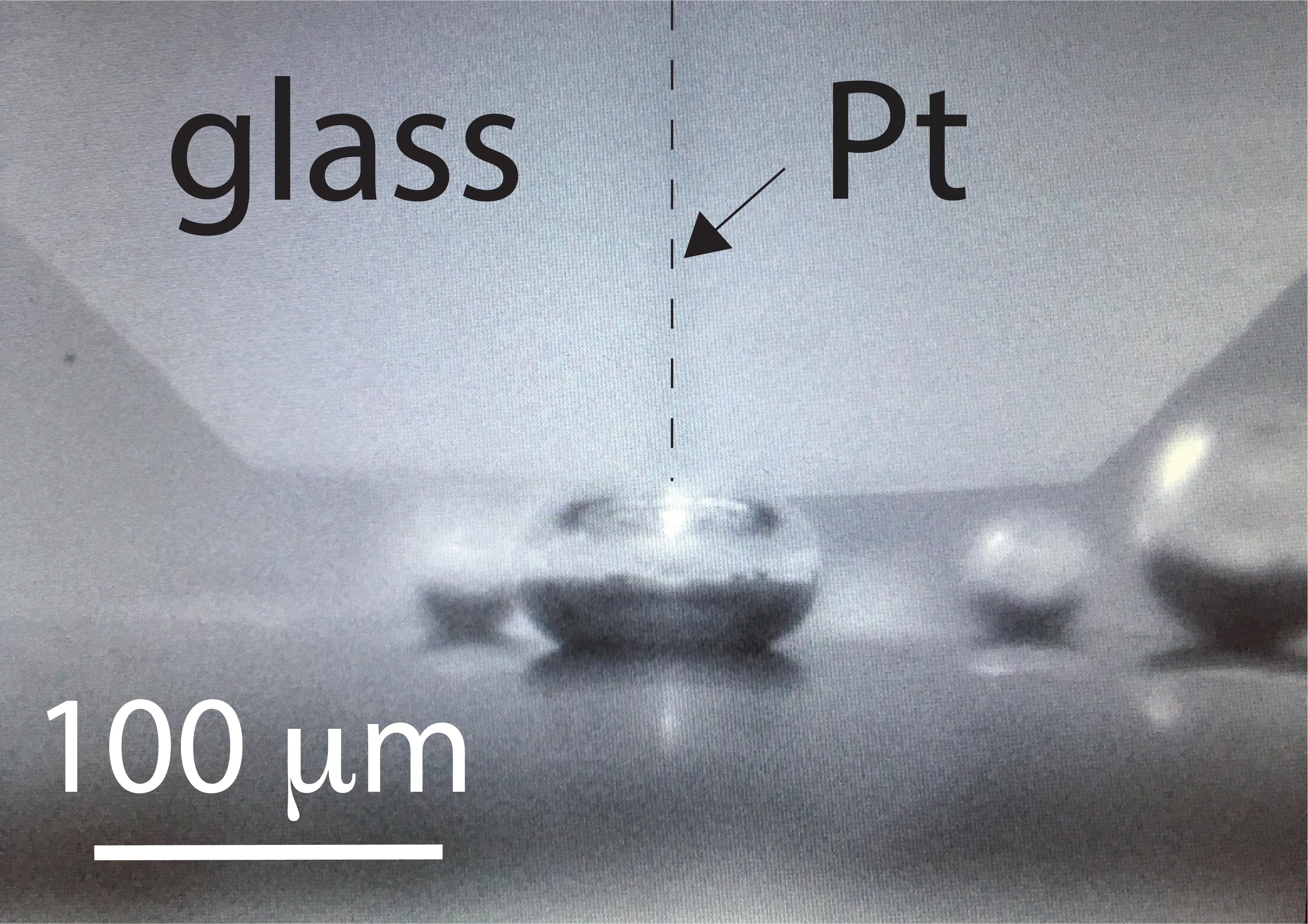 Shortly after these first experiments, I received a visit from Avni Berisha from the University of Pristina. We agreed that his Ph.D. student, Veton Haziri, could help me explore the electrochemical signature of gas bubbles in greater detail. Veton spent one year in my research laboratory under the ERASMUS+ exchange program. He, alongside a project student (Tu Phan Tran Nha), collected the experimental data that led to our article, now published in Communications Chemistry (link to paper here).
Shortly after these first experiments, I received a visit from Avni Berisha from the University of Pristina. We agreed that his Ph.D. student, Veton Haziri, could help me explore the electrochemical signature of gas bubbles in greater detail. Veton spent one year in my research laboratory under the ERASMUS+ exchange program. He, alongside a project student (Tu Phan Tran Nha), collected the experimental data that led to our article, now published in Communications Chemistry (link to paper here).
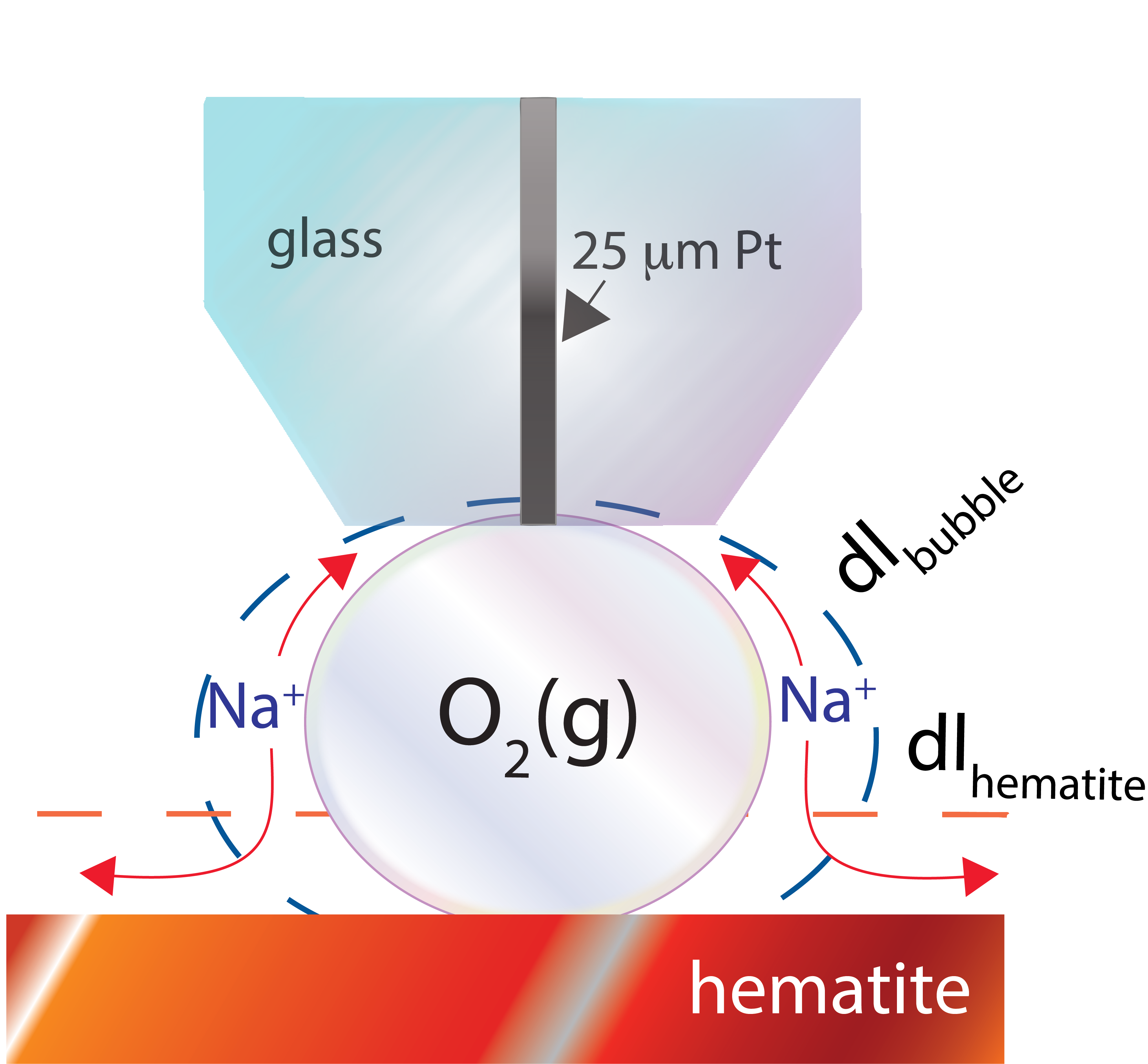
We here show that gas bubbles are more than simple vehicles for gas transport. When pinned on solid surfaces, they establish an electrochemical gateway enabling the exchange of ions. As bubble surfaces are negatively charged, due to hydroxyl ions to the air/water interface, only counteractions can be exchanged. We show that this gateway is possible where gas bubbles are pinned on solid surfaces of contrasting composition, such as hematite or gold. This gateway is closed for bubbles pinned on surfaces of similar in composition, such as Teflon which also stabilises hydroxyl ions in a comparable manner to the air/water interface. We even show that bubble impedance can be directly altered by electric potentials applied through the supporting solid. In particular, negative electric potentials enhance cation transport at bubble surfaces.
We believe that this gateway could play an important role in electric shielding and reaction overpotentials caused by bubbles on catalysts. It could even be intrinsically tied to the ability of bubbles to produce hydroxyl radicals, such as reported in a recent paper by Vogel et al. (Nat. Comm. 2020) that we found only shortly after correcting the proofs of this article. This gas bubble-driven (electro)chemistry could have especially important ramifications for predicting processes including mineral flotation, microfluidics, pore water geochemistry, and fuel cell technology.
You can read out paper "A gateway for ion transport on gas bubbles pinned onto solids" here.
Harizi V, Nha TPT, Berisha A, Boily JF. 2021. A gateway for ion transport on gas bubbles pinned onto solids. Comm. Chem. 4, 43.
https://www.nature.com/articles/s42004-021-00481-7
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in