Iron-catalysed C–H/C–H coupling for dimeric to polymeric thiophene materials synthesis
Published in Chemistry
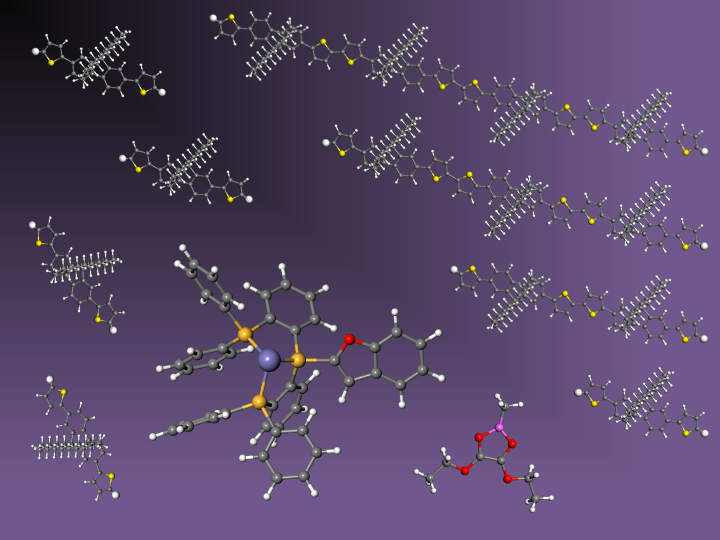
Dimeric to polymeric thiophene compounds have wide application in electronic devices such as organic field-effect transistors, organic light-emitting diodes, and organic solar cells. Currently, these compounds are synthesized using transition-metal-catalysed cross-coupling reactions, but there are several disadvantages: preinstallation of C–M (M: metal) and C–X (X: (pseudo)halide) moieties to the substrates and removal of those moieties from the product in the case of polymerization (Fig. 1).
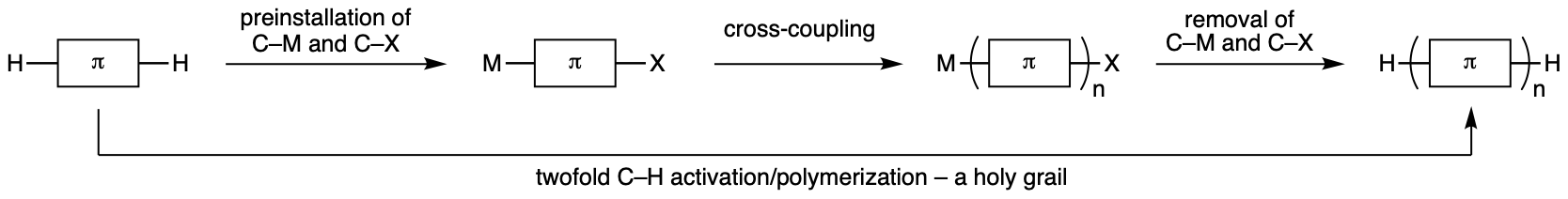
Figure 1. Transition-metal-catalysed C–H/C–H coupling for polymerization—a holy grail
Therefore, thienyl C–H/C–H coupling has attracted much attention as an ideal method to synthesize dimeric to polymeric bithiophene compounds. However, strongly oxidizing conditions are often required for the formal removal of two hydrogen atoms, which limit substrate versatility and reaction selectivity. For example, the classical reactions through cationic or radical cationic mechanisms (e.g., the Scholl reaction and oxidative aromatic coupling)[1] are limited in scope, producing isomers and undesired side products.
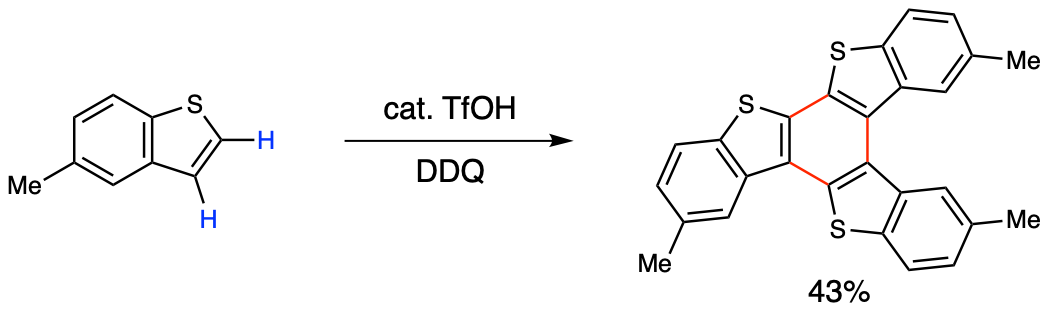
Figure 2. Thienyl C–H/C–H coupling through cationic or radical cationic mechanisms
A modern variation using palladium-catalysed C–H activation has broadened the scope,[2] but the requirement of an oxidant (e.g., Cu(II) or Ag(I)) to turn over the Pd(II)/Pd(0) cycle with a large redox potential [E°(PdII/Pd0) = +0.915 V vs NHE] has limited its synthetic versatility, especially in the synthesis of redox-sensitive, electron-rich, and highly conjugated molecules.
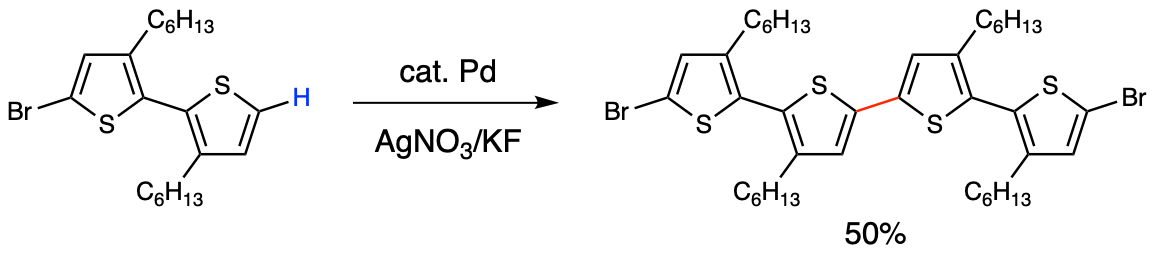
Figure 3. Palladium-catalysed thienyl C–H/C–H coupling
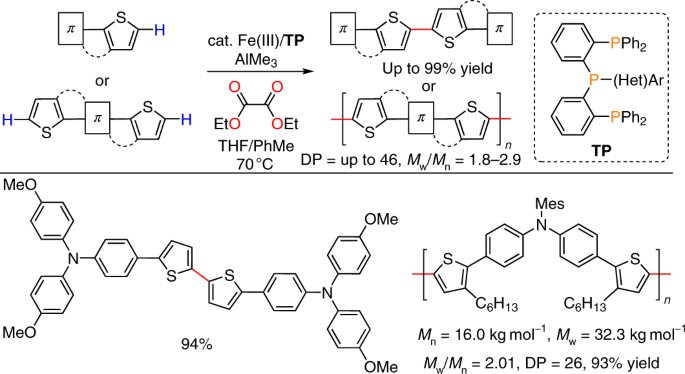
In this work, we report iron-catalysed regioselective thienyl C–H/C–H coupling using conjugated tridentate phosphine (TP) as a ligand, AlMe3 as a base, and diethyl oxalate (DEO) as a mild two-electron acceptor, enabling the synthesis of -conjugated dimeric, oligomeric, and polymeric thiophene compounds of importance in materials science.

Figure 4. Iron-catalysed regioselective thienyl C–H/C–H coupling
To accomplish this work, there were two big challenges: 1) Finding a mild oxidant that selectively turns over the iron catalytic cycle and 2) Development of new TP ligands that suppress catalyst deactivation and enable polycondensation. The breakthrough for the first challenge was the observation that alkene generated by two-electron reduction of dihaloalkane, which we have been using as an oxidant for iron-catalysed C–H activation for over 10 years, inhibits the iron catalysis by its stronger coordination ability than that of the thiophene substrate. This prompted us to investigate other mild oxidants that do not generate alkene throughout the course of the reaction. Because the previous reaction that we reported using TP ligand was ortho C–H methylation of aromatic ketones and we knew that ketones can effectively coordinate to iron,[3] we came up with the idea of using diketone as a mild oxidant. The strong oxophilicity of Al(III) contributes as the driving force for effective catalyst turnover (Fig. 5). After examining various kinds of diketones, we found that diester, or oxalate, serves as an excellent oxidant for iron-catalysed thienyl C–H/C–H coupling. With this mild oxidant in hand, various kinds of redox-sensitive thiophene compounds that can be used as organic materials for organic devices were synthesized by thienyl C–H/C–H coupling in high to excellent yields.
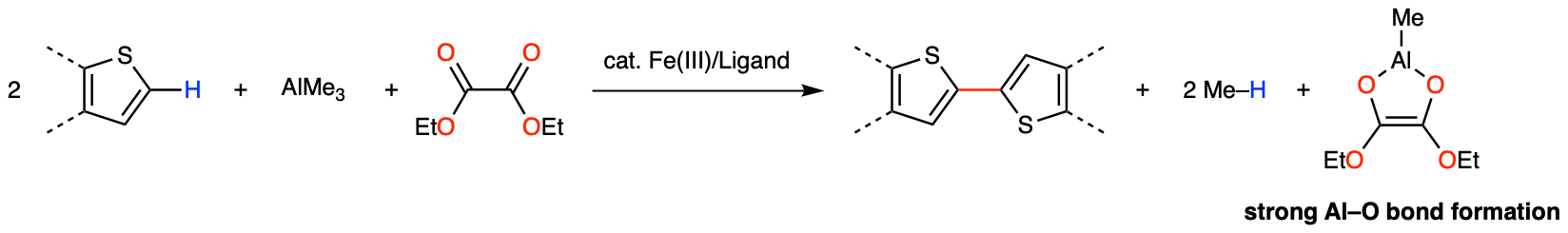
Figure 5. Iron-catalysed regioselective thienyl C–H/C–H coupling using oxalate as a mild oxidant
The breakthrough for the second challenge was the finding that the TP ligand is methylated through intramolecular C–H activation during the reaction, causing catalyst deactivation. Because of this catalyst deactivation, phenyl-substituted TP ligands were not effective for thienyl C–H/C–H condensation, which requires extreme catalytic activity. To prevent ligand methylation, we designed heteroaryl-TPs where the central phosphine is substituted with a heteroarene. Based on the report that unsymmetrically substituted tertiary phosphines can be synthesized by sequential addition of sterically hindered organolithium reagents to triphenyl phosphite,[4] we have successfully prepared indolyl-TP, benzofuryl-TP, and benzothienyl-TP by simple experimental procedures. After examining the effect of these ligands on thienyl C–H/C–H coupling, we have determined benzofuryl-TP to be the best ligand, and the polymer length was doubled compared with the case of phenyl-substituted TP. This method allows us to synthesize polymeric semiconductive materials of interest in materials science from easily synthesized thiophene monomers. The termini of the polymers are truncated with a hydrogen atom, hence obviating the end-capping procedure that is typically required in the conventional optoelectronic polymer synthesis (e.g., polymerization by C–M/C–X coupling or C–H/C–X coupling) to remove the potentially detrimental halide or organometallic termini for device applications. Because of the weak interaction of Fe(III) with the polymer’s π surface, the polymerization took place through a step-growth mechanism and the residual catalyst was easily removed by posttreatment of the polymer by a thiol-functionalized silica scavenger. This work highlights the benefits of using two earth-abundant metals, iron and aluminum, for the synthesis of π-conjugated small and macromolecules of importance in energy device applications.[5]
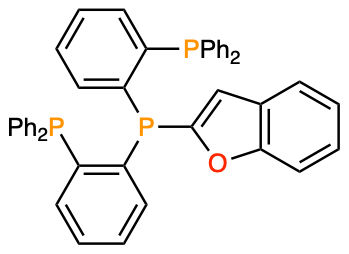
Figure 6. Development of heteroaryl-TP for iron-catalysed thienyl C–H/C–H polycondensation
In summary, this work highlights the benefits of cooperative use of two earth-abundant metals, iron and aluminum, for the synthesis of π-conjugated small and macromolecules of importance in organic electronic device applications. We consider that the structural and electronic elements built in the TP ligand and in the DEO/Al(III) combination have struck a good balance for the control of the valence and the spin state of organoiron intermediates. The C–H/C–H polycondensation represents an addition to a still limited repertoire of organoiron catalysis in polymer synthesis, and will serve as a model for further development of practically useful iron catalysts.
Please find our manuscript published in Nature Catalysis here: https://doi.org/10.1038/s41929-021-00653-7.
If you are interested in other works by our group, please take a look at our group website: https://moltech.jp/ja/.
(Takahiro Doba thanks Dr. Rui Shang and Prof. Eiichi Nakamura for their helpful advice for preparing this post.)
References
[1] Grzybowski, M., Skonieczny, K., Butenschön, H. & Gryko, D. T. Comparison of oxidative aromatic coupling and the Scholl reaction. Angew. Chem. Int. Ed. 52, 9900–9930 (2013).
[2] Yang, Y., Lan, J. & You, J. Oxidative C–H/C–H coupling reactions between two (hetero)arenes. Chem. Rev. 117, 8787–8863 (2017).
[3] Shang, R., Ilies, L. & Nakamura, E. Iron-catalyzed ortho C–H methylation of aromatics bearing a simple carbonyl group with methylaluminum and tridentate phosphine ligand. J. Am. Chem. Soc. 138, 10132–10135 (2016).
[4] Keller, J., Schlierf, C., Nolte, C., Mayer, P. & Straub, B. F. One-pot syntheses of sterically shielded phosphorus ligands by selective stepwise nucleophilic substitution at triphenyl phosphite. Synthesis 2006, 354–365 (2006).
[5] Nakamura, E. & Sato, K. Managing the scarcity of chemical elements. Nat. Mater. 10, 158–161 (2011).
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in