Iron oxide nanozymes stabilize stannous fluoride for targeted biofilm killing and synergistic oral disease prevention
Published in Biomedical Research

Dental caries, commonly known as tooth decay, is an alarming global health concern, affecting more than 3.1 billion individuals and resulting in annual costs of over $290 billion1, 2. Dental caries arises from the formation of pathogenic biofilm, which provides a safe haven for microorganisms. Within the biofilm, microenvironments with acidic pH levels form, accelerating the growth of acidogenic bacteria and subsequently leading to tooth enamel acid dissolution. Although fluoride treatment has been our primary defense against tooth decay3, it does not entirely prevent disease, especially among high-risk individuals or those with reduced saliva flow4, 5, because of limited effects on biofilms. Overexposure to fluoride can also lead to adverse effects, such as dental fluorosis, especially in young children6, 7.
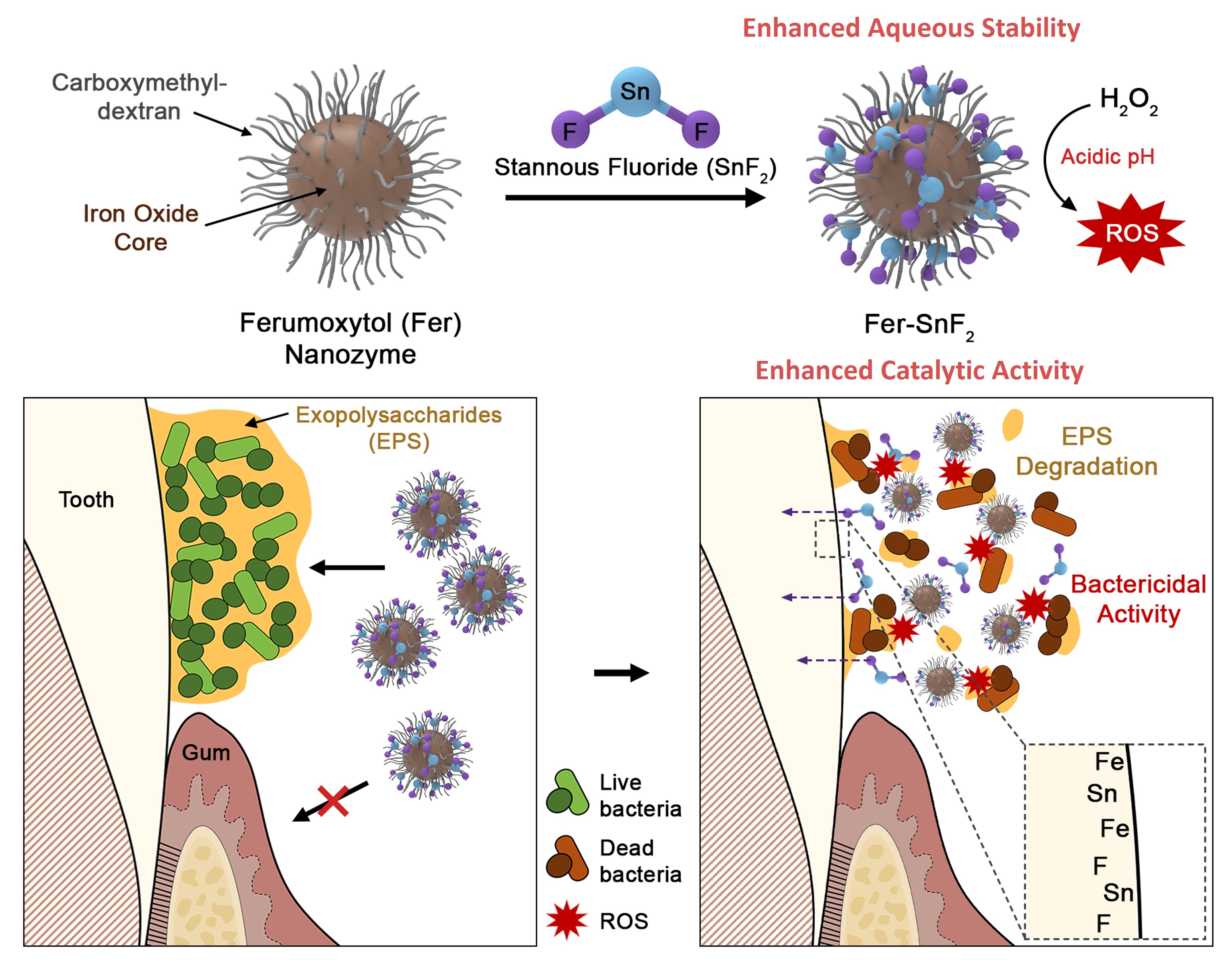
Ferumoxytol (Fer) is an FDA-approved iron oxide nanoparticle formulation initially designed for treating iron deficiency. Our previous studies have demonstrated its potent effects against oral biofilms when applied topically8, 9. The action primarily involves selectively binding to pathogens and generating reactive oxygen species (ROS) in acidic conditions due to its peroxidase-like activity. However, it doesn't fully halt the progression of disease. This led us to an intriguing hypothesis: could a combination of Fer and fluoride amplify the therapeutic benefits without increasing fluoride exposure?
We investigated this by combining Fer with two widely available fluoride formulations: sodium fluoride (NaF) and stannous fluoride (SnF2). Interestingly, while the combination with NaF did not show improvement, the mixture of Fer and SnF2 yielded potent synergistic results, enhancing antibiofilm efficacy. Notably, SnF2, often hampered by stability issues in aqueous solutions, was remarkable stable when mixed with Fer. Further examination revealed that the Sn2+ from SnF2 was bound to the carboxylate groups in Fer’s carboxymethyl-dextran coating. This is particularly noteworthy since commercial SnF2 formulations require chemical additives for stability10.
Subsequent analysis led to another interesting finding. While SnF2 alone did not produce any ROS, its combination with Fer amplified Fer's catalytic activity. Factors like pH, concentration, and incubation time affected the enhancement of ROS production by SnF2, with the most pronounced activity at pH 4.5. This indicates its potential to target the bacteria causing dental caries under acidic pH microenvironments. Animal studies using a rodent model revealed that this combined treatment not only prevented dental caries but also entirely halted enamel cavitation, which is a milestone yet to be achieved by any other treatment. Importantly, these results were achieved at dosage four times lower than typical dosages, potentially alleviating concerns about fluoride overexposure.
Furthermore, traces of fluoride, iron, and tin were detected on the enamel's outer layers forming a protective film. This suggests that the combination therapy might provide lasting protection against enamel demineralization, introducing a revolutionary mechanism for caries prevention.
To this end, the significance of this discovery lies in its dual therapeutic action to address both the pathogenic biofilm and providing enamel protection, while simplifying formulation development due to enhanced stability. Because severe tooth-decay in children is often linked with iron deficiency anemia, the combination of Fer and SnF2 could represent the next frontier in oral care that address two major health problems, tooth decay and anemia, warranting further exploration and clinical trial.
The combination of Fer and SnF2 brings forth an innovative, synergistic strategy against dental caries. As oral health remains a global concern, the combined use of Fer and SnF2 offers a potent and feasible solution without increasing fluoride exposure.
References
- Peres, M.A., Macpherson, L.M.D., Weyant, R.J., Daly, B., Venturelli, R., Mathur, M.R., et al., Oral diseases: a global public health challenge. Lancet. 394, 249-260 (2019)
- Kassebaum, N.J., Bernabe, E., Dahiya, M., Bhandari, B., Murray, C.J., & Marcenes, W., Global burden of untreated caries: a systematic review and metaregression. J. Dent. Res. 94, 650-658 (2015)
- Featherstone, J.D., Prevention and reversal of dental caries: role of low level fluoride. Community Dent. Oral Epidemiol. 27, 31-40 (1999)
- Koo, H., Allan, R.N., Howlin, R.P., Stoodley, P., & Hall-Stoodley, L., Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 15, 740-755 (2017)
- Hicks, J., Garcia-Godoy, F., & Flaitz, C., Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). J. Clin. Pediatr. Dent. 28, 47-52 (2003)
- Wong, M., Clarkson, J., Glenny, A-M., Lo, E., Marinho, V., et al., Cochrane reviews on the benefits/risks of fluoride toothpastes. J. Dent. Res. 90, 573-579 (2011)
- Whelton, H.P., Spencer, A.J., Do, L.G., & Rugg-Gunn, A.J., Fluoride revolution and dental caries: evolution of policies for global use. J. Dent. Res. 98, 837-846 (2019)
- Liu, Y., Naha, P.C., Hwang, G., Kim, D., Huang, Y., Simon-Soro, A., et al., Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat. Commun. 9, 2920 (2018)
- Liu, Y., Huang, Y., Kim, D., Ren, Z., Oh, M.J., Cormode, D.P., et al., Ferumoxytol nanoparticles target biofilms causing tooth decay in the human mouth. Nano Lett. 21, 9442-9449 (2021)
- Myers, C.P., Pappas, I., Makwana, E., Begum-Gafur, R., Utgikar, N., Alsina, M.A., et al., Solving the problem with stannous fluoride: Formulation, stabilization, and antimicrobial action. J. Am. Dent. Assoc. 150, S5-S13 (2019)
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in