Irradiation and lithium treatment alter the global DNA methylation pattern and gene expression underlying a shift from gliogenesis towards neurogenesis in human neural progenitors
Published in Neuroscience

The rate of childhood brain tumor incidence varies among countries; there are 1.15 - 5.14 cases per 100,000 children, with the United States reporting the highest rate [1]. In Sweden, 100 children per year are diagnosed with a brain tumor. Over 80% of them overcome the dreadful disease, mainly with the help of brain radiation therapy, but not without consequences [2].
Radiation therapy uses high-energy rays to treat cancer. It works by damaging the cancer cells and making it hard for them to regenerate. Your body then is naturally able to ”get rid” of these damaged cancer cells. Yet, there is still much room for improvement. The same life-saving radiation therapy that kills cancer cells, unfortunately also damages normal, healthy cells and has many life-long side effects. As many as 95.7% of children treated for a brain tumor experience some form of persistent late effects in adulthood [2]. The side effects include decline in cognition and mood, decreased social competence, and fatigue.
There are currently around 11 thousand children or young adults in Sweden who have beaten brain cancer, but still their lives are far from perfect. We can no longer just focus on curing cancer, but must also prevent and treat the complications. A leading hypothesis to explain the decline in cognition, at least partially, is that the radiation therapy causes injury to the neural stem and progenitor cells (NSPCs), the cells which will give rise to neurons and other brain cell types, like glial cells, which leads to cell death and altered fate choice, favoring gliogenesis over neurogenesis. For this reason, treatments harnessing neurogenesis are of great relevance in this context.
Lithium, a well-known mood stabilizer, used for the treatment of bipolar disorder, has neuroprotective and antitumor effects, and has, in previous studies from the Blomgren and Hermanson labs, been found to reverse irradiation-induced damage in rodents, associated with regulation of the expression of the glutamate decarboxylase 2 gene (Gad2) via promoter demethylation in rat NSPCs. Additionally, lithium was shown to rescue irradiation-induced cognitive defects in mice, with the use of behavioral tests [3]. It was previously not known if the same neuroprotective effects of lithium after irradiation would be translatable to a human system.
![Figure 1. Neurogenesis is an essential process to proper brain development and irradiation causes major disruptions. Whole brain irradiation depleted the proliferating (DCX+) cells in the granule cell layer on post-natal-day (PND) 91 mice, 2 weeks after lithium discontinuation. DCX+ cell density was decreased by irradiation, but this decrease was reversed by lithium treatment. Created in BioRender.com and adapted from Zanni et al, 2021 [3].](https://images.zapnito.com/cdn-cgi/image/metadata=copyright,format=auto,quality=95,fit=scale-down/https://images.zapnito.com/uploads/62JNuBMZQqemE0rUOHtf_blog_picture%201.png)
Figure 1. Neurogenesis is an essential process to proper brain development and irradiation causes major disruptions.
Whole brain irradiation depleted the proliferating (DCX+) cells in the granule cell layer on post-natal-day (PND) 91 mice, 2 weeks after lithium discontinuation. DCX+ cell density was decreased by irradiation, but this decrease was reversed by lithium treatment. Created in BioRender.com and adapted from Zanni et al, 2021 [3].
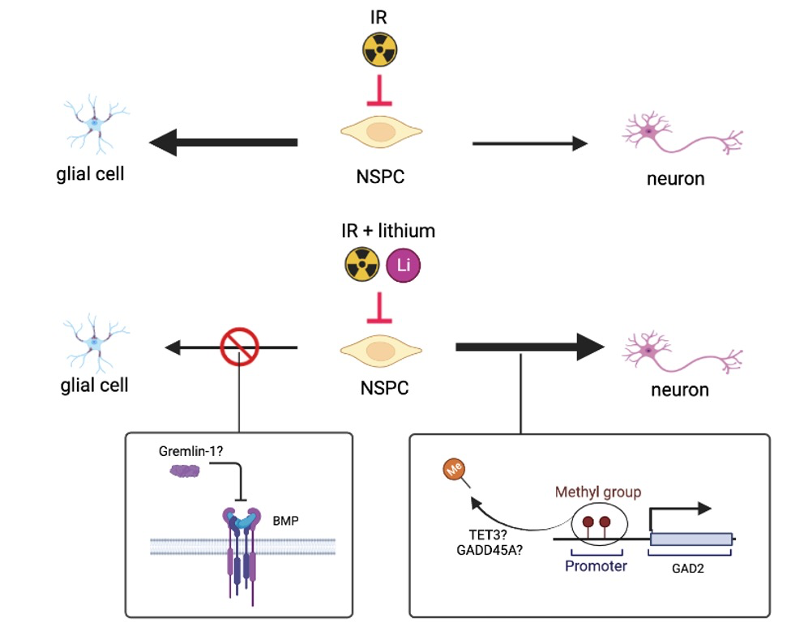
In our most recent study, we show that irradiation (IR) alone or in combination with lithium chloride (LiCl) caused major changes in gene expression and global DNA methylation in stem cell-derived human NSPCs (hNSPCs) compared to untreated cells, as well as LiCl-only-treated cells. Here, we demonstrated that irradiation altered the DNA methylation profile of hNSPCs, poising gene promoter regions important for transcription factor binding, an effect which was further specified after lithium treatment. Several studies have established that lithium promotes NSPC survival and proliferation post-irradiation [3, 4, 5, 6, 7]; our data suggest that the effect of lithium in irradiated human NSPCs is dependent on epigenetic regulation.
As mentioned, key pathology of the irradiated hippocampus is a skewed developmental process that favors gliogenesis over neurogenesis [3]. Post-irradiative lithium treatment led to increased demethylation of several genes related to a functional neurogenesis and negative control of gliogenesis. The promoter demethylation was also accompanied by transcriptional upregulation of developmentally important genes, refocusing this developmental equilibrium towards neurogenesis, such as GAD2, and away from gliogenesis, such as GREM1, SOX10, SOX5 and PTN, which taken together can be central to the positive effects of lithium. The promoters of these genes were found to be differentially methylated in irradiated and lithium treated cells, with patterns consistent with their expression profiles compared to the other experimental groups. This selective mechanism of methylation alterations after IR + LiCl treatment may be controlled by TET3 and GADD45A, two proteins known to be methylation regulators, since the genes encoding for them were found to be upregulated after irradiation and lithium treatment in neural progenitor cells compared to the control.
Lithium is presumed to have a range of still poorly understood mechanisms through which it restores damage of the irradiated brain [3, 6]. Shining a light on the epigenetic role of lithium in favoring neurogenesis over gliogenesis in irradiated human neural progenitors further strengthens the arguments for its clinical use. Based on these data, as well as previous findings from the Blomgren and Hermanson lab [3], a phase II, randomized, placebo-controlled, double-blind trial assessing lithium treatment in cranial radiation therapy-treated pediatric brain cancer survivors, LiBRA, has been approved by the Swedish Ethics Review Board and the Swedish Medical Products agency on May 2nd 2023, and will commence at the Astrid Lindgren Children’s Hospital after the summer, led by Klas Blomgren.
Cover image credits: Photo by National Cancer Institute on Unsplash
References
- Johnson KJ, Cullen J, Barnholtz-Sloan JS, Ostrom QT, Langer CE, Turner MC, et al. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiol Biomarkers Prev. 2014 Dec;23(12):2716-36.
- Lönnerblad, M, van't Hooft, I, Blomgren, K, Berglund, E. A nationwide, population-based study of school grades, delayed graduation, and qualification for school years 10-12, in children with brain tumors in Sweden. Pediatr Blood Cancer. 2020; 67:e28014.
- Zanni G, Goto S, Fragopoulou AF, Gaudenzi G, Naidoo V, di Martino E, et al. Lithium treatment reverses irradiation-induced changes in rodent neural progenitors and rescues cognition. Molecular Psychiatry. 2021 Jan 14;26(1):322–40.
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000 Oct;75(4):1729–34.
- Zanni G, di Martino E, Omelyanenko A, Andäng M, Delle U, Elmroth K, et al. Lithium increases proliferation of hippocampal neural stem/progenitor cells and rescues irradiation-induced cell cycle arrest in vitro. Oncotarget. 2015 Nov 10;6(35):37083–97.
- Zhou K, Xie C, Wickström M, Dolga AM, Zhang Y, Li T, et al. Lithium protects hippocampal progenitors, cognitive performance and hypothalamus-pituitary function after irradiation to the juvenile rat brain. Oncotarget. 2017 May 23;8(21):34111–27.
- Huo K, Sun Y, Li H, Du X, Wang X, Karlsson N, et al. Lithium reduced neural progenitor apoptosis in the hippocampus and ameliorated functional deficits after irradiation to the immature mouse brain. Mol Cell Neurosci. 2012 Aug;51(1–2):32–42.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Feb 28, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in