Is there a scientific way to preserve cadmium red paints in twentieth century paintings? A proposed spectroscopic answer
Published in Physics

Cadmium red is the name used to designate a class of 20th century artists’ pigments described by the general chemical formula CdS1-xSex, whose color can be synthetically tuned from red-orange to red-purple with increasing selenium content (Fig. 1). For their vibrant hues and excellent covering power, a number of renowned modern and contemporary painters, including Piet Mondrian, Gerardo Dottori, Joan Mirò, Jackson Pollock, Nicolas de Staël, made an extensive use of cadmium reds.
As direct band gap semiconductors, CdS1-xSex compounds show a peculiar luminescence both in the visible and in the near infrared regions that changes according to their selenium content, permitting to distinguish among different varieties of the pigment in paintings by means of completely non-invasive diagnostic techniques. Likewise other artists’ pigments, cadmium reds are suspected to be prone towards color change. Thus, with the final aim to optimize preventive conservation strategies for invaluable artworks, it becomes fundamental to find an answer to the following question: "What are the intrinsic properties of CdS1-xSex and environmental agents influencing the luminescence and stability of cadmium reds in paintings?"
In 2022, our paper, published in The European Physical Journal Plus, we have answered this question. In this post, we lead you through the experimental pathway of our investigation.
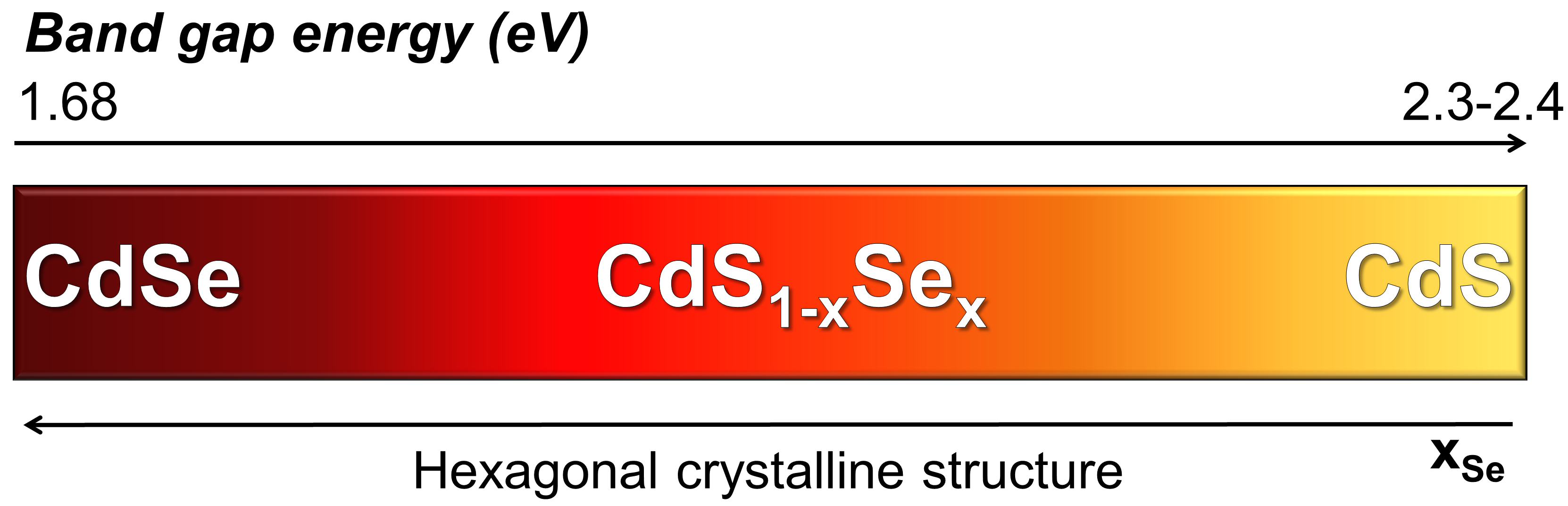
Figure 1. Illustration of the structural, chemical, optical and electronic properties of different cadmium-based compounds used as artists’ pigments.
The luminescence properties and fading of cadmium reds
CdS1−xSex semiconductor pigments exhibit direct radiative recombination with green-orange emission in the 530-630 nm range (known as near band edge emission, NBE) and radiative deactivation from intragap trapping states due to crystal defects, which give rise to two peculiar red-near infrared emissions in the 780-1100 nm range (known as deep level emissions, DLEs). The positions of the NBE and DLEs as well as that of the inflection point of the diffuse reflectance visible spectra mainly depend on the selenium content of CdS1−xSex; therefore, photoluminescence and diffuse reflectance vis–NIR spectroscopy have been profitably used for the non-invasive identification of different cadmium red varieties in paintings over the last decade. The origin of the DLE bands and the factors affecting their relative intensity is however still a matter of debate. Babentsov et al. have tentatively assigned the presence of the two DLE bands to two different types of cation–anion divacancies (VCd-VSe) in CdSe. Brasil et al. have instead suggested that the occurrence and relative intensity of the DLE1 and DLE2 bands is attributable to the concentration of lattice-vacancy type defects (VSe) and oxygen-containing species incorporated in the vacancies, respectively.
The phenomenon of cadmium reds changing color has been investigated in the field of heritage science. The fading of red-orange CdS1-xSex has been documented in recent times by Rayner et al. only in an ancient Greek terracotta krater, where the pigment was used for restoration purposes. In this case, light and chlorine species were proposed as two possible triggering factors of the alteration.
Our paper
In our article, to gain new insights in the environmental agents (i.e. light and relative humidity, RH) and internal factors influencing the photoluminescence properties and (photo)chemical reactivity of cadmium red paints, we benefitted from a combination of macro-scale vis-NIR and vibrational spectroscopies with micro-/nano-scale advanced electron microscopy mapping and X-ray methods employing conventional and synchrotron radiation sources, namely X-ray powder diffraction and X-ray absorption spectroscopy [the latter performed at beamlines ID21 and LISA of ESRF (Grenoble, France)].

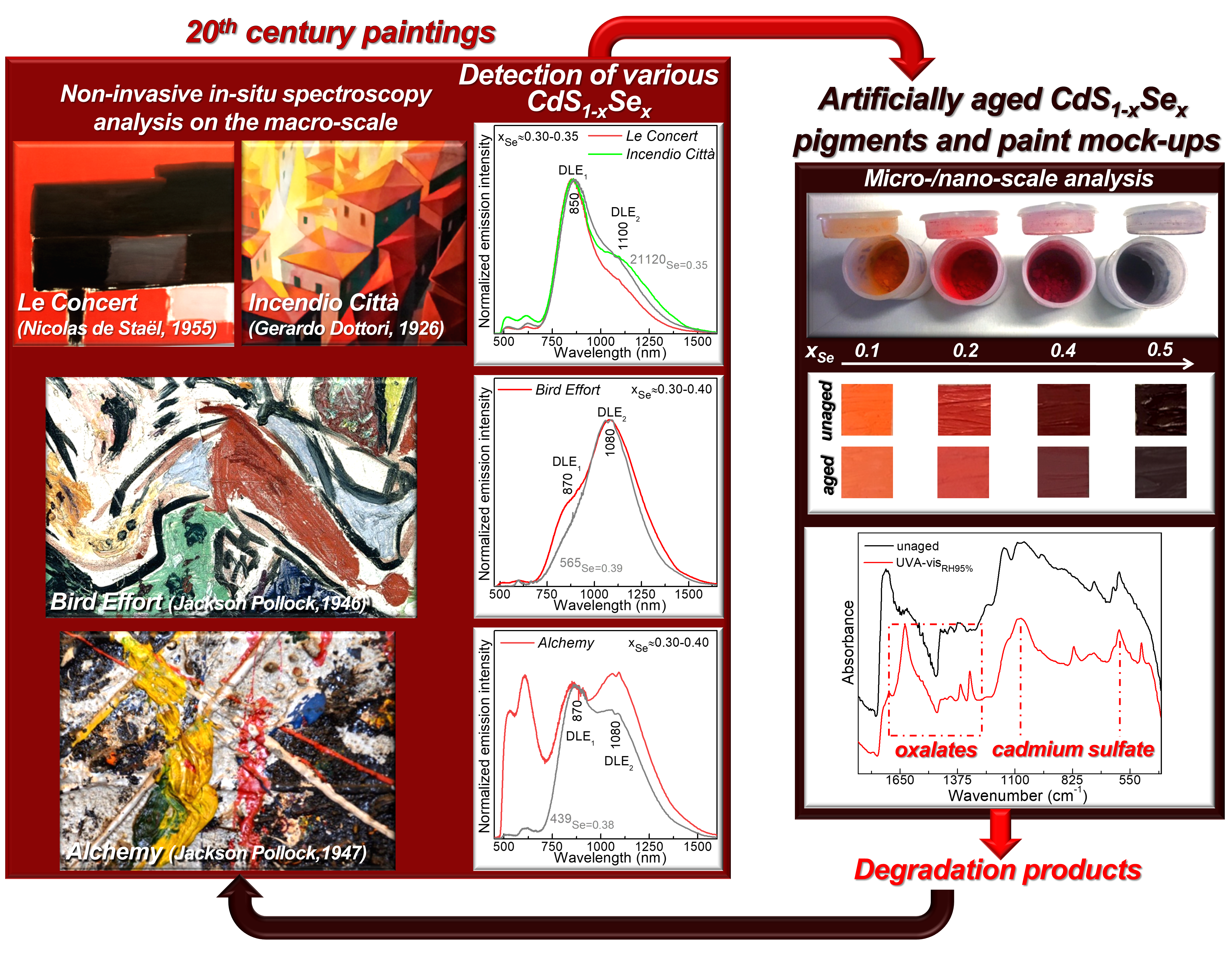
Figure 2. Photographic details of the cadmium red paints
where CdS1-xSexwith different selenium content and
showing different luminescence properties were identified.
Paintings by J. Pollock belong to the Peggy Guggenheim
Collection of Venice (Italy), while that by G. Dottori is owned
by the Civic Museum of Palazzo della Penna of Perugia (Italy).
Non-invasive vis-NIR spectroscopy data obtained using the portable equipment of the MOLAB platform of E-RIHS from nine masterpieces by Dottori, Pollock and de Staël allowed classifying the CdS1-xSex-paints in three groups, according to the relative intensity of the two DLE bands (Figures 2, 3).
These outcomes, combined with results from micro-/nano-scale electron microscopy mapping and X-ray analysis of a set of CdS1-xSex powders and artificially aged paint mock-ups, demonstrated that the relative intensity of DLEs is not affected by the morphology, microstructure and local atomic environment of the pigment particles but it is influenced by the presence of moisture. Furthermore, the results obtained from the extensive study of artificially aged paint mock-ups made up of CdS1-xSex pigments with a variable Se content (with 0.1<x<0.5) permitted us to provide first evidence of the tendency of cadmium reds toward photo-degradation and to prove that the conversion of CdS1-xSex to cadmium sulfate and/or oxalates is more efficient for the selenium-poorer CdS1-xSex types and is triggered by high moisture conditions and by the presence of the oil binder.
The combination of the findings from paint-mock-ups with the results from the non-invasive spectroscopy investigations of selected cadmium red-based areas of Alchemy and Bird Effort by J. Pollock led us to conclude that the observed cadmium sulfate and cadmium oxalate can be interpreted as alteration products of the two analyzed historical paintings (Figure 3).
Looking forward
The experimental findings of this study contribute to the optimization of preventive conservation strategies of twentieth century paintings containing cadmium reds. The alteration of this class of pigments results in a fading of the paint surface that is difficult to perceive with the naked eye; however, attention should be paid to such a degradation phenomenon, since it promotes the formation of more mobile and (partially) soluble compounds, such as sulfates. Notably, the fading of cadmium red paints can be mitigated using conditions as earlier proposed for another class of cadmium pigments, cadmium yellows, namely:
- minimization of the exposure of the painting to moisture (i.e., RH%<40%);
- maintenance of illumination conditions at the regular values foreseen by the Commission Internationale de l´Eclairage (CIE) for lightfast painting materials.
Further research is ongoing to expand knowledge on how other factors related to the manufacturing process of cadmium reds may affect their peculiar photoluminescence and to investigate and clarify the way in which selenium participates to the degradation pathways of CdS1-xSex paints, via the possible formation of oxidized selenium-compounds.
Follow the Topic
-
The European Physical Journal Plus

This journal encompasses all aspects of fundamental and applied physics, including energy, environment, cultural heritage, research infrastructures and citizen science, and welcomes in particular interdisciplinary topics.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in