Is this really a 2D conductive polymer (polyaniline)?
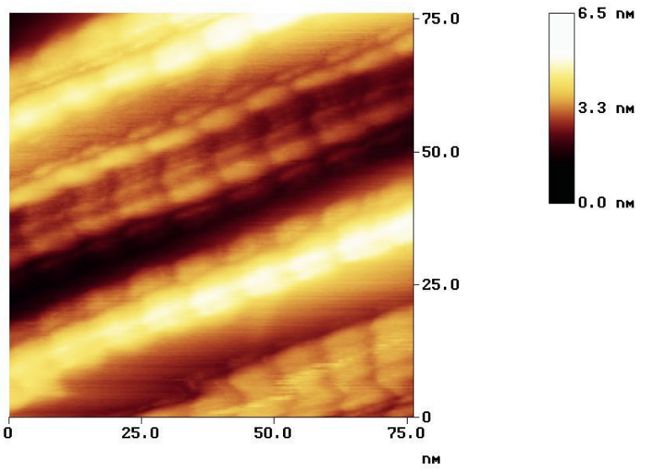
When I became aware of Zhang's et al. paper in "nature", I was excited: the authors announced a "fundamental breakthrough" in understanding the structure / conductivity relationship of conducting polymers, polyaniline. I had worked most of my professional life in this area, I wanted to learn more.
However, I was disappointed after I had the chance to read the paper and the additional material. One reason for this was their claim to have found an extraordinarily high conductivity never seen for polyaniline, which is not correct. So I contacted the first author, and he admitted not to have seen, hence not having cited my own papers showing even a much higher conductivity.
However more important was, that I could not see how they derived their exciting and really beautiful structure model: ok, the transmission electron microscopy showed a nice arrangement of atoms, but what exactly was its chemical structure? There was no real chemical analysis, their µXPS results did not match the claimed chemical structure at all, and the paper did not explain why the chemical process (not being fundamentally different from what is shown in most polyaniline related papers) should lead to a completely different structure than seen before - not that this structure and especially the 2D arrangement was not interesting, in contrast, highly interesting: but why could it be built that way, why did the chemical analysis not fit with the claimed structure, why did the colour (i.e. the visual manifestation of a UV-VIS spectrum, not shown) not fit with regular conductive polyaniline, but with its oxidized (non-conductive) form pernigraniline,
and: why had the absolute conductivity values been by a factor of 10 lower than our own record values, and, even more important, qualitatively not at all different from what we had shown in Fig 3. in one of our studies? This is very important, because our polyaniline was and is not crystalline, and it consists of globular (in fact: ellipsoidal, i.e. clearly 3D) primary particles of about 6*13 nm small size?
This is not (only) a question of usual, normal conduct for scientific publications, but in this case a fundamental question: Why would this 2D monolayer polyaniline represent a fundamental breakthrough while the absolute conductivity is much lower than what can be achieved in technical, in fact: in industrial scale, and the conductivity characteristic (conductivity vs temperature) the same as with 3D polyaniline which is commercially available?
Especially when looking at the chemical synthesis which takes 48 hours and requires very careful removal of the ultrathin layer from the reaction liquid, leading to an extremely low yield?
After I had discussed this with the authors and after I had sent a comment to "nature" intended to be published as "matters arising", I had not got any further answers, and "nature" has rejected my comment paper which you may find here as preprint.
So, to my regret, my questions shown here (and in other form with more details in my preprint) are remaining unanswered.
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.
Ask the Editor – Polymers
Got a question for the editor about Functional polymers? Ask it here!
Continue reading announcement




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in