ISRCTN's Rear View Mirror - 2025 in review
Published in Healthcare & Nursing, Biomedical Research, and General & Internal Medicine

A record-breaking year
Last year, more than 1,600 new clinical studies were registered with ISRCTN, making 2025 our busiest year yet. October broke our record for most studies published in one month, before November immediately smashed this again.
Despite the deluge of submissions, we’re proud that our turnaround times haven’t just remained unscathed, but improved along the way. Following the launch of our new submission form (more on that later!), November’s median time from submission to registration was just five days.
We also piloted a project in September to measure our response times after a new submission reaches us. Across the first nine months of 2025, over 99% of submissions received an initial editorial assessment and response within one working day.
A new look
We debuted our new logo, created by Oxford PharmaGenesis, and added a tagline - 'The UK's Clinical Study Registry'. The logo design represents the centralisation of clinical study data to make research more transparent, and the tagline makes our shared mission clearer. We also streamlined the site to make it clearer to navigate, allowing users to get help and guidance more easily.

ISRCTN’s 25th birthday
In April we celebrated the 25th anniversary of ISRCTN by inviting the researchers behind the 10,000th, 15,000th and 20,000th study registrations to discuss their research, covering topics including inguinal hernias, acute asthma and COVID-19.
We also created a timeline looking back at the history of ISRCTN and the key milestones in its development and an infographic of key facts and figures.
.png) |
.png) |
Expanding our reach
Earlier in the year, we published an interactive map showing all the UK locations where ISRCTN-registered studies have originated from over the years.
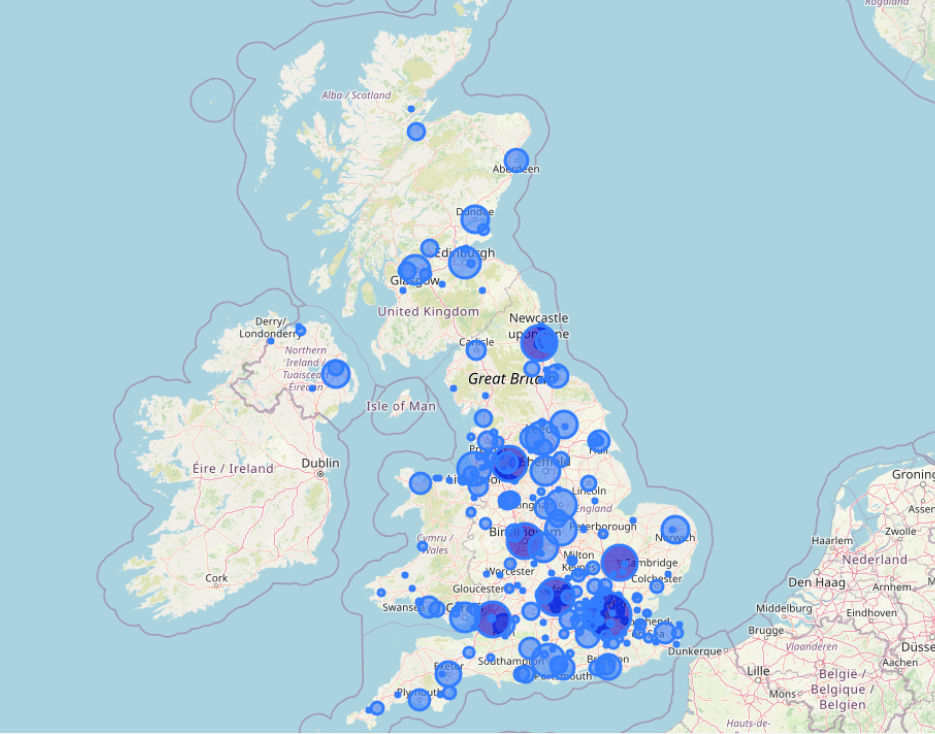
As always, in addition to being the UK’s Clinical Study Registry, we’re proud to accept submissions from anywhere worldwide. In 2025, we have registered clinical studies recruiting patients from 255 nations.
ISRCTN in the news
We found ourselves in the spotlight several times in 2025, within different industry publications which have featured ISRCTN prominently as a component for encouraging transparent, robust research in the UK and beyond.
In May, Pink Sheet discussed the Health Research Authority’s annual trial registration report. Senior Writer Vibha Sharma noted that while many UK researchers still opt for ClinicalTrials.gov, ISRCTN’s new submission system could draw in a wider audience into 2026.
Later in the year, Lauren Nardella reported on updated HRA guidance recommending ISRCTN, for our “alignment with national transparency goals and integration with UK systems”.
Meanwhile on TranspariMED, Till Bruckner covered insights into plans for two of our upcoming projects – creating a comprehensive overview of all UK clinical studies, and a call for input from researchers on the design of a new results reporting system during last summer.
Research Communities: highlights from 2025
Researchers who register their studies at ISRCTN are invited to write blog posts about their work for the Springer Nature Research Communities to raise awareness of their trials and discuss them in more detail. But what were the top 3 most read blog posts submitted by researchers in 2025?
In July, Simon Göbel et al. posted a blog about the first clinical trial (WAVES) on the effects of drinking water quantity and quality, looking specifically at the effects of hydration on the rehabilitation outcomes of critically ill patients.
In May, Ifunanya Clara Agu and her colleagues wrote a blog post about their study improving youth-friendly sexual and reproductive health services through gender transformative and intersectional approaches in Nigeria, a project that aligns closely with the United Nations’ Sustainable Development Goals (SDGs), particularly SDG 3 and SDG 5.
Jasmine Laing discussed her research interests for International Nurses Day as part of our SDG 3 awareness days campaign, including the SHAPE study of cognitive and behavioural coaching to support health and social care workers living with depression and posttraumatic stress disorder (PTSD) in the UK.
Research making headlines
We’re always excited to see research registered with ISRCTN making the news. Back in 2018, we registered details of the Big Baby Trial, where pregnant mothers expecting particularly large offspring were given the option to be induced a couple of weeks early at 103 hospitals across the country.
Results were published in The Lancet earlier in 2025, showing that early delivery reduced the risk of shoulder dystocia, where the baby’s shoulder becomes stuck during birth. BBC News then covered the story, focusing on the mothers whose experience of childbirth was benefited through being given the option of induction at 38 weeks.
News coverage does not always wait for results to be available: In July, The Independent reported on a brand new clinical trial being set up at the Southampton Clinical Trials Unit, to help detect ten types of cancer in their early stages. ISRCTN had registered this study in February, and we’re always hopeful that early press coverage like this will lead to more patients discovering new trials and getting involved – in this case the researchers are hoping to recruit 1000 participants to be screened.

Other ISRCTN-registered studies picked up in the news this year have focused on Huntington’s disease, mobile phones in schools, rheumatoid arthritis, leukaemia and physical activity in children.
What have the ISRCTN editorial team been up to in 2025?
As well as our editorial duties, the ISRCTN editorial team runs projects focused on easing the registrant’s journey to complete registration. We do this through bimonthly team discussions on salient topics, social media output, and conference attendance, all while keeping up with changing registration guidance.
One of our well-read blog posts of the year offered a behind-the-scenes look at the work that underpins every ISRCTN record. In From Submissions to Support: A Day in the Life at ISRCTN, a Registry Editor shared a candid snapshot of a typical working day, from the first coffee-fuelled triage of new submissions to the careful review of study records against WHO registry requirements.
The post highlighted the editorial detail involved in checking study design, ethical approval and transparency, as well as the collaborative support provided to study teams responding to queries and managing updates.
We are keenly aware of the role of patients in the studies that are registered. Making research accessible to everyone is central to ISRCTN’s mission. By supporting the NIHR Be Part of Research platform with plain English summaries, ISRCTN helps ensure people don’t need a medical background to understand clinical studies. Our editors work with researchers to explain what a study involves, why it matters, and what taking part could mean, using clear, everyday language. This emphasis on transparency helps build trust, empowers patients and families to make informed choices, and makes clinical research feel more open, relevant and approachable.
Up there with the proudest moments of the year has got to be the November release of the streamlined ISRCTN submission form. The ISRCTN team developed the new form in collaboration with our stakeholders and developers to help registrants apply to register their studies with the core WHO 24 minimum required data fields. Thus, paving the way for an equally streamlined editorial process and their studies being considered fully registered in record breaking time.
ISRCTN’s presence at RDF25 reinforced our commitment to equity in clinical research. Reflections shared by the Programme Manager captured key discussions on the persistent lack of diversity in study participation and the need to engage underserved communities from the very start of study design. These conversations continue to shape how ISRCTN supports more inclusive and transparent research across the UK.
What’s coming in 2026?
Significant changes to UK clinical trials law and policy were also announced in 2025, strengthening expectations around transparency and study registration. Updated clinical trials regulations, developed by the MHRA in partnership with the Health Research Authority (HRA), place increased emphasis on timely public registration and clearer reporting of study information.
New HRA guidance is helping researchers and sponsors prepare for these requirements ahead of the regulations coming fully into force in April 2026, reinforcing the role of ISRCTN as a public registry in making research more visible, accountable and accessible.
Follow the Topic
-
ISRCTN: The UK’s Clinical Study Registry

A primary clinical trial registry recognised by WHO and ICMJE that accepts studies involving human subjects or populations with outcome measures assessing effects on human health and well-being, including studies in healthcare, social care, education, workplace safety and economic development.
Your space to connect: The Primary immunodeficiency disorders Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine, Immunology, and Diseases!
Continue reading announcement




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in