Iterative sublattice amorphization facilitates exceptional processability in inorganic semiconductors
Published in Materials
Plastic inorganic semiconductors: a new interdisciplinary field
In the context of emerging wearable devices, sensors, and power sources with flexibility have been in great demand. However, inorganic semiconductors are generally brittle at room temperature due to their directional covalent bonds, which will break under external bending, stretching, or curving, severely blocking their usage in flexible electronics. With the discovery of plastic Ag2S in 2018, a burgeoning interdisciplinary of plastic inorganic semiconductors has been generated, which could endure exceptional plastic deformation without fracture.
In the past few years, despite newly reported plastic inorganic semiconductors, the underlying deformation mechanism that differs these materials from brittle ones is still elusive, especially for Ag2Te1-xSx solid solutions possessing metal-like ductility, which have attracted our attention and motivated the research in the past years.
Sublattice amorphization during plastic deformation
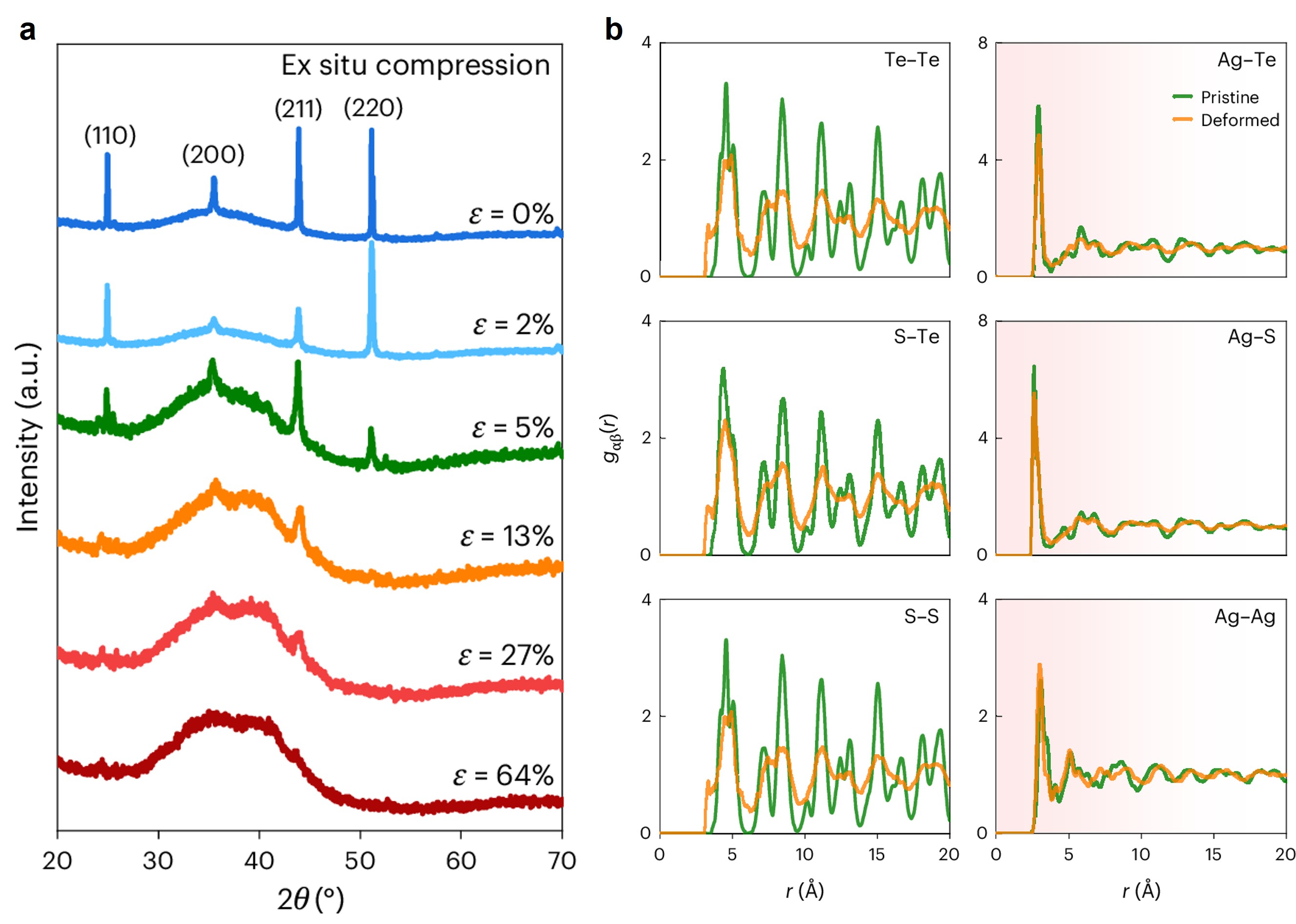
Ag2Te1-xSx (0.3 ≤ x ≤ 0.6) solid solutions are superionic conductors at room temperature, where the Te/S anions form cubic sublattice, and Ag cations are distributed randomly among the rigid sublattice. For metals where dislocations dominate plastic deformation, their crystalline structures are unchanged during deformation. However, an unusual amorphization is surprisingly observed in Ag2Te0.6S0.4 after a compression test in this work, implicating the potential interrelationship between plastic deformation and amorphization. The ex-situ compression-XRD experiments (Fig. 1a) along with in-situ TEM tests reveal that the crystalline structures of Ag2Te0.6S0.4 gradually become amorphous with increasing compressive strain from both macro- and micro-scale. Interestingly, even slight polishing could lead to surface amorphization in Ag2Te1-xSx (0.3 ≤ x ≤ 0.6), which is quite different from the general cases in metals where severe deformation is required to induce amorphization.
The pair distribution function (PDF) by synchrotron radiation along with the reverse Monte Carlo (RMC) simulation is used to analyze structural configurations (Fig. 1b). The reduction of peak intensity and broadening of peak shape of partial PDFs between anion pairs demonstrate an obvious amorphization in Te/S sublattice after deformation. In contrast, the almost unchanged partial PDF shapes of Ag-Te/S pairs suggest the Ag ions are always distributed randomly, as the result of its superionic characteristic, which ensures the bonding between Te/S and Ag atoms always exists. Therefore, when subjected to external force, the Te/S sublattice gradually becomes amorphous to accommodate plastic strain, while the randomly distributed Ag ions keep bonding with Te/S atoms to ensure the integrity of the structure. The molecular dynamics (MD) simulations further verify this deformation mechanism.
Iterative sublattice amorphization induced metal-like processability
Sublattice amorphization endows Ag2Te0.6S0.4 with deformability. However, with constant consumption of crystalline structures to accommodate strain, only amorphous structures are left, and the sample would finally break, evidenced by the breakage in cold-rolling operations. An ingenious strategy of iterative sublattice amorphization transition is designed, where amorphous structures can be tuned back into the crystalline ones before the occurrence of fracture. As a result, an ultrahigh extensibility of about 10150% is achieved in cold-rolling through four times iterative sublattice amorphization transition (Fig. 2a). Interestingly, the transport properties are almost unchanged during deformation (Fig. 2b). Such strips by rolling could be easily cut into variable shapes, and fabricated as flexible thermoelectric (TE) devices (Fig. 2c). Besides rolling, other traditional metal processing methods, including drawing and forging can also be applied in Ag2Te0.6S0.4 at room temperature by the iterative sublattice amorphization strategy, facilitating the mass-production and low-cost fabrication of flexible modules.
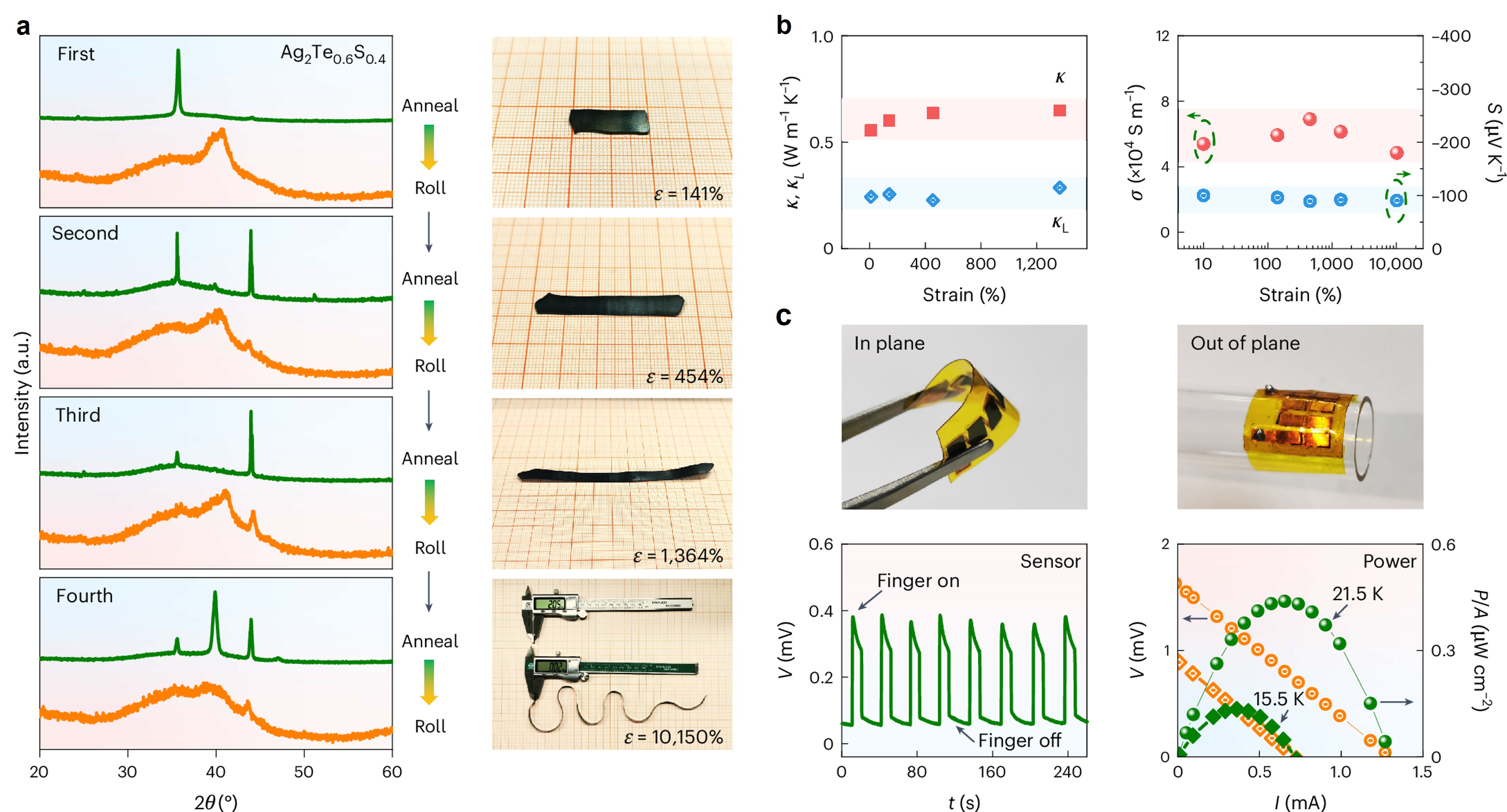
Fig. 2. (a) Extraordinary processibility achieved in Ag2Te0.6S0.4 by iterative sublattice amorphization strategy. (b) The stable transport properties during deformation. (c) The obtained flexible thermoelectric devices and performance.
What’s going on?
This publication reveals the unique deformation mechanism in Ag2Te1-xSx (0.3 ≤ x ≤ 0.6) inorganic semiconductors and achieves metal-like processibility at room temperature. Whether this sublattice amorphization be expanded to other materials? Or could it be a guide to explore/design new plastic inorganic semiconductors? Moreover, with the discovery of new plastic inorganic semiconductors, is it possible to summarize a common deformation mechanism for them? These possibilities remain for further exploration.
Follow the Topic
-
Nature Materials

A monthly multi-disciplinary journal that brings together cutting-edge research across the entire spectrum of materials science and engineering, including applied and fundamental aspects of the synthesis/processing, structure/composition, properties and performance of materials.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in