Journal Club | Chaos under pressure
Published in Chemistry
In a recent Communications Chemistry article, researchers show that the properties of high-entropy oxides (HEOs) can be modulated through pressure loading. The results improve our fundamental understanding of these materials that are already used in Li-ion batteries, high-performance capacitors, or in CO2 fixation.
So what’s special about HEOs? In this material class, a single solid solution lattice is carefully messed up with additional atoms, until a multi principle component structure is reached. The occupancies of atomic positions in the solid solutions are random, which maximizes the configurational entropy, which in turn stabilizes the chaotic system. The surprising thing to note here is that this element jumble works also for an ionic lattice with located partial charges, as in HEOs. Unsurprisingly, there still is a lot to learn about such complex systems.
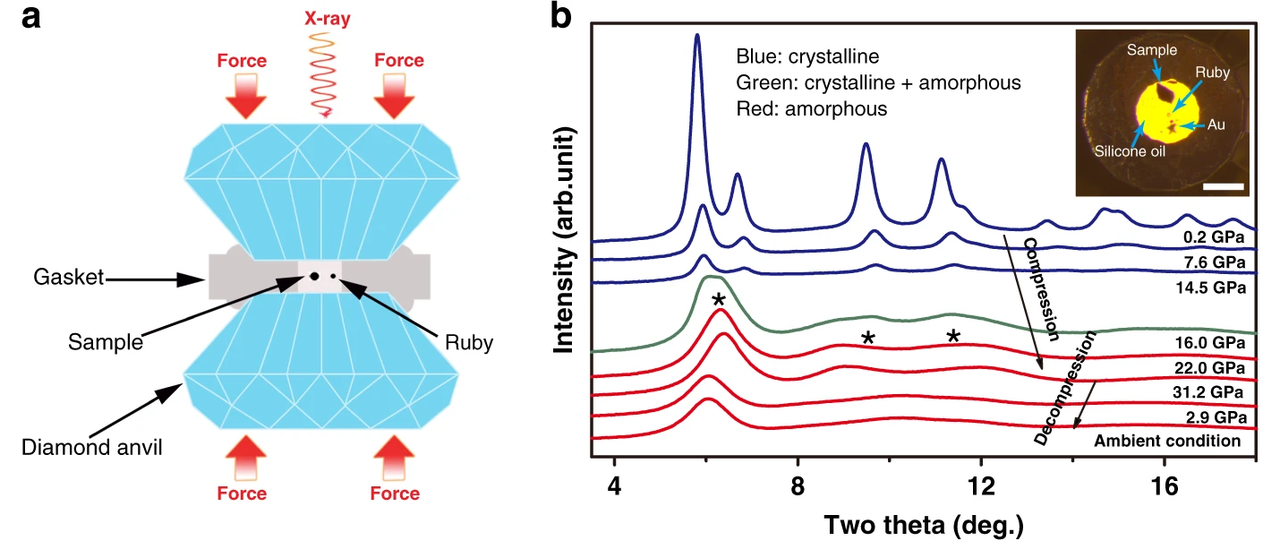
Qiaoshi Zeng and co-authors observe the single-crystal structure of (Ce0.2La0.2Pr0.2Sm0.2Y0.2)O2−δ while it is deforming under pressure. After producing the material through nebulized spray pyrolysis, undoubtedly a lit synthesis technique, the authors use in situ synchrotron diffraction as well as vibrational spectroscopy to identify lattice distortions.
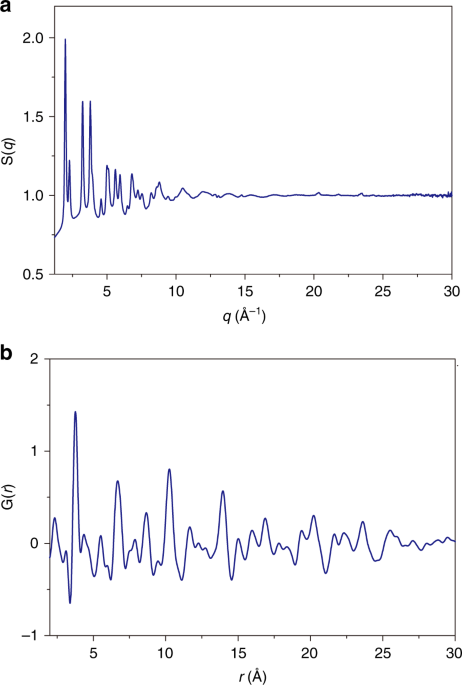
The applied pressure of up to roughly 30 GPa eventually causes the oxide to amorphize. During compression, the initial CaF2-type structure gradually loses its crystal coherence, indicated by peak broadening. Interestingly, the REO8 cubes in this HEO are quite rigid and remain almost constant during compression, while all the other longer atomic distances between different REO8 cubes considerably decrease with increasing pressure. This long-range coherence eventually breaks down when the pressure exceeds the mechanical instability limit. The resulting amorphous-like phase exists up to the highest applied pressure and is kinetically retained during decompression. Hence, as the material partially maintains its amorphous state, a glass-nanoceramic composite HEO is obtained.
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in