Journal Club | Macroevolution of offspring size in vertebrates mediated by life history
Published in Ecology & Evolution

In general, competitive animals like dinosaurs develop toward gigantism, since this is advantageous in seizing habitats, preying behavior, and courtship (Apaldetti et al. 2018). Larger offspring are critical to population competition, especially where resources are relatively scarce. The relationship of offspring size to adult size partially reflects the competitive capacity of the species. This is the main tenet of Density Hypothesis, which posits that the relative size of offspring represents their resource acquisition ability (Falster et al. 2008). However, for evolutionary biologists, the intrinsic determinants or the underlying mechanism of the scaling exponents (slopes) of the offspring size at independence to their parents remain unclear.
Rollinson et al (2019) used a large dataset of vertebrate body size that span fishes, amphibians, reptiles, birds, and mammals to disentangle the variance sources in determining the scaling exponents. They classified several specific clades in fishes and amphibians, which differed markedly from previous studies that set them as a single clade. For example, fish lineages were classified as Cyprinodontiformes and Perciformes, while amphibians included Anura and Urodele.
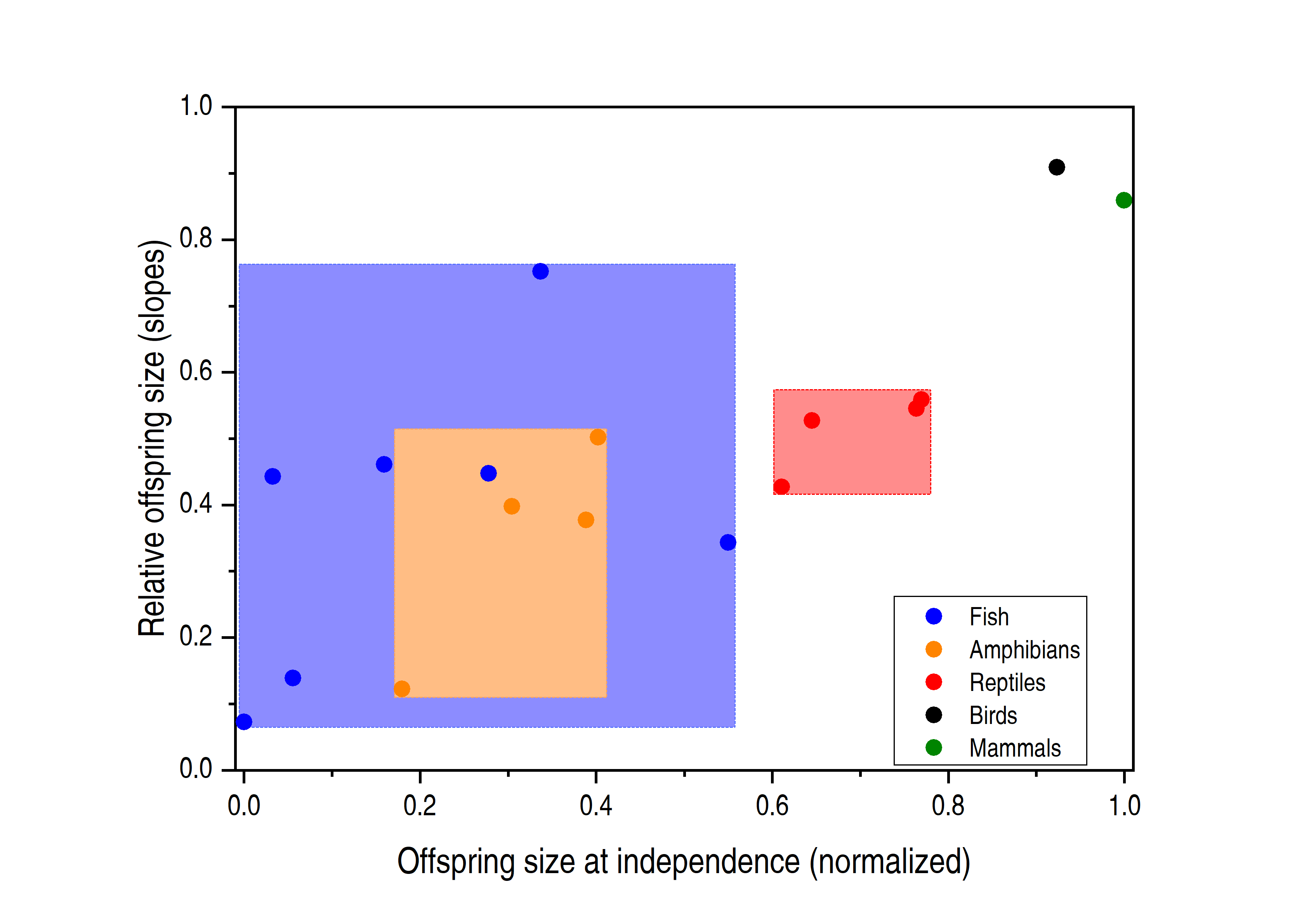
Figure 1. Relationship between the relative offspring size and the offspring size at independence. The colored dots correspond to lineages, and the relatively lighter colored areas indicate the distribution of data points, showing that fish and amphibians have a large overlapping area. The data points are from the original figure 3 of Rollinson et al, which is normalized to offspring size at independence by the maximum and minimum values of the original data points.
Two interesting patterns emerged when comparing the seven clades. First, in fish, amphibians, birds, and mammals, the offspring to adult sizes did increase overall, with the scaling exponents increasing as the evolution position increased (Fig. 1), consistent with a previous study (Falster et al. 2008). However, when analyzing the data in the same lineage of fish and amphibians, the authors found that the slopes of the offspring size fluctuated significantly. For example, Perciformes fishes that spawn demersally exhibited a steeper slope than pelagic-spawning Perciformes, and direct developing Anurans exhibited a significantly steeper slope than did Anurans species with larval development. These observations contradict the Density Hypothesis, which predicts similar slopes in demersally and pelagic-spawning fishes because both may face similar competition environments. Direct developing Anurans can live on land and escape the intra-species competition, and thus yield lower slopes, which is contradictory.
Rollinson et al then analyzed the types in which next generations grow. They believed that the offspring size that cannot strictly follow the evolutionary route may be related to the strategy of avoiding competition. Indeed, for some vertebrates that generally protect eggs and embryos, such as direct-developing frogs, demersal-spawning fishes, and viviparous animals, the ectotherms always have larger offspring than congeners that do not protect their offspring (Pollux et al. 2014). Rollinson et al described that Cyprinodontiformes fishes with matrotrophic viviparity exhibited a steeper slope than either lecithotrophic viviparous or oviparous species (Fig. 1). This surpasses most amphibians and reptiles, even though these fishes are considered as lower evolutionary lineages. These observations underscore that parental care is crucial in determining the relative offspring size.
Based on the data concerning relative size and life history of the vertebrates’ offspring, Rollinson et al developed a new model. In this model, relative offspring size can be 52% explained by the absolute size of the offspring, which is a fairly high proportion. The model shows that larger animals have inherent evolutionary advantages, and indicates that animals may adjust their evolutionary position by changing their life history. For example, the large overlap between fish and amphibians and reptiles (Fig. 1) suggests that some fishes can adopt the viviparous route, yielding larger offspring than amphibians and reptiles. As for the remaining unexplained variation, the authors speculated that pre-maturation growth is important. For example, the offspring of domesticated mammals are larger than the wild (Milla et al. 2018), due to slower metabolic rates and longer maturity duration. In addition, the habitat environment's transformation of life history (Esquerré et al. 2019; Gao et al. 2019) and nest predation (Kubelka et al. 2018) may direct impact offspring size. This requires future study.
In summary, this study considers the impact of life history on offspring size in fish and amphibians. Future research should focus on the impact of life history of birds and mammals. Conducting similar studies can help us to better understand the biological similarity and unity of organisms and to disentangle the underlying mechanisms of size evolution in vertebrates.
LITERATURE CITED
Apaldetti, C., R. N. Martínez, I. A. Cerda, D. Pol, and O. Alcober. 2018. An early trend towards gigantism in Triassic sauropodomorph dinosaurs. Nat. Ecol. Evol. 2:1227–1232.
Esquerré, D., I. G. Brennan, R. A. Catullo, F. Torres‐Pérez, and J. S. Keogh. 2019. How mountains shape biodiversity: The role of the Andes in biogeography, diversification, and reproductive biology in South America’s most species‐rich lizard radiation (Squamata: Liolaemidae). Evolution 73:214–230.
Falster, D. S., A. T. Moles, and M. Westoby. 2008. A General Model for the Scaling of Offspring Size and Adult Size. Am. Nat. 172:299–317.
Gao, W., Y.-B. Sun, W.-W. Zhou, Z.-J. Xiong, L. Chen, H. Li, T.-T. Fu, K. Xu, W. Xu, L. Ma, Y.-J. Chen, X.-Y. Xiang, L. Zhou, T. Zeng, S. Zhang, J.-Q. Jin, H.-M. Chen, G. Zhang, D. M. Hillis, X. Ji, Y.-P. Zhang, and J. Che. 2019. Genomic and transcriptomic investigations of the evolutionary transition from oviparity to viviparity. Proc. Natl. Acad. Sci. 116:3646–3655.
Kubelka, V., M. Šálek, P. Tomkovich, Z. Végvári, R. P. Freckleton, and T. Székely. 2018. Global pattern of nest predation is disrupted by climate change in shorebirds. Science 362:680–683.
Milla, R., J. M. Bastida, M. M. Turcotte, G. Jones, C. Violle, C. P. Osborne, J. Chacón-Labella, Ê. E. Sosinski, J. Kattge, D. C. Laughlin, E. Forey, V. Minden, J. H. C. Cornelissen, B. Amiaud, K. Kramer, G. Boenisch, T. He, V. D. Pillar, and C. Byun. 2018. Phylogenetic patterns and phenotypic profiles of the species of plants and mammals farmed for food. Nat. Ecol. Evol. 2:1808–1817.
Pollux, B. J. A., R. W. Meredith, M. S. Springer, T. Garland, and D. N. Reznick. 2014. The evolution of the placenta drives a shift in sexual selection in livebearing fish. Nature 513:233–236.
Rollinson, N., V. Nilsson‐Örtman, and L. Rowe. 2019. Density‐dependent offspring interactions do not explain macroevolutionary scaling of adult size and offspring size. Evolution evo.13839.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in