Ketamine's antidepressant effects involve an evolutionarily ancient part of the brain
Published in Neuroscience and General & Internal Medicine

Why ketamine?
Ketamine, commonly used as a dissociative anesthetic, is an effective antidepressant drug1. It has several interesting properties: it works quickly (within hours to a small number of days), has sustained effects after a single dose, and seems to be effective at treating depression in people who have not responded to several other treatments (called treatment resistant depression, or TRD). Ketamine also is effective in improving symptoms of anhedonia, defined as a lack of interest or pleasure in things usually found pleasurable2. This can otherwise be resistant to treatment with conventional antidepressant drugs. These properties mean there is a lot of interest in understanding how ketamine works at a neurobiological level.
Why the anterior cingulate cortex?
The anterior cingulate cortex (ACC) is one brain region implicated in the antidepressant effects of ketamine3. The ACC is an evolutionarily ancient area of cortex wrapping around the corpus callosum, a large bundle of white matter connecting the two hemispheres of the brain. It is involved in a myriad of functions, including attention, decision-making, reward processing, emotion regulation and autonomic function. The heterogeneity in function reflects the fact that the ACC consists of distinct subdivisions defined relative to their position around the genu (‘knee’) of the corpus callosum (Figure 1; genu labelled ‘g’). Each subdivision has a distinct cellular architecture, numbered as Brodmann areas (BAs) after the German anatomist Korbinian Brodmann, who defined them4. The dorsal (d)ACC sits above the genu (BA24 & 32); the perigenual (pg)ACC around the genu (BA24 & 32); and the subgenual (sg)ACC ventral to the genu (including BA24, 25 and 32).
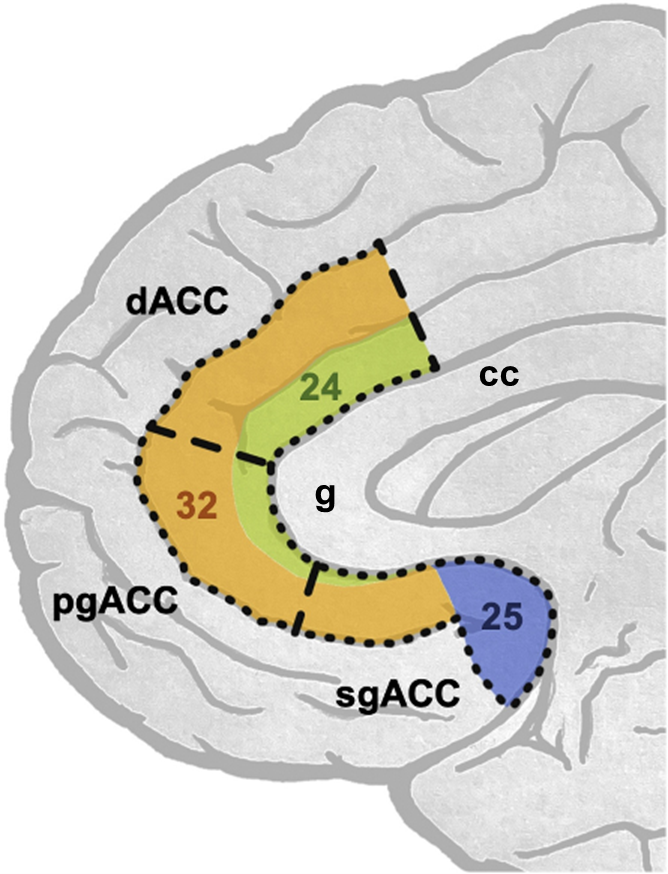
Functional neuroimaging studies in healthy volunteers (HVs) and in people with depression implicate functional changes within the ACC as being important in ketamine’s effects, but the precise location varies across studies. We had previously reviewed literature suggesting that modulation of ACC activity is critical in mediating ketamine’s antidepressant effects, and we wanted to test this hypothesis3.
How did we explore ketamine’s effects on the ACC?
Through open data sharing between King’s College London (KCL, UK) and the National Institute of Mental Health (NIMH, USA), we performed a secondary data analysis on existing neuroimaging data to test the effects of ketamine on the connectivity of ACC subregions. Resting-state functional magnetic resonance imaging (fMRI) had been collected data from a double-blind, randomized, placebo-controlled crossover trial, exploring the effects of intravenous infusions of ketamine compared to placebo (saline) in people with TRD compared to HVs (Figure 2)5,6.

We wanted to see how the regions of the ACC were affected by ketamine compared to placebo, and test whether these effects were different between those with TRD compared to the non-depressed group. We chose sgACC, pgACC and dACC as our three regions of interest, and measured changes in resting-state functional connectivity (rsFC) from these regions to the whole brain two days after the respective infusions. rsFC is a measure of correlated fluctuations in activity between different brain areas, and changes in rsFC are therefore thought to reflect changes in the functional relationships between these areas. The study also included measures of depression & anhedonia symptoms, so we could investigate whether changes in connectivity correlated with changes in symptoms.
What did we find?
Comparing the TRD group to the non-depressed group, ketamine differentially affected how the ACC subregions connected with the anterior ventromedial prefrontal cortex and the right insula cortex – brain regions implicated in the regulation of emotion and internal bodily states (Figure 3A). Compared to placebo, ketamine-induced changes in connectivity between the pgACC and the right insula cortex correlated with the improvements in depression symptoms (Figure 3B). Given these changes were measured two days after infusion, it would suggest that plastic alterations in pgACC connectivity are important in the sustained antidepressant effects of ketamine, enabling ketamine to exert an antidepressant effect even when it is no longer present in the circulation. The comparison with non-depressed controls suggests this effect is specific to the depressed state.
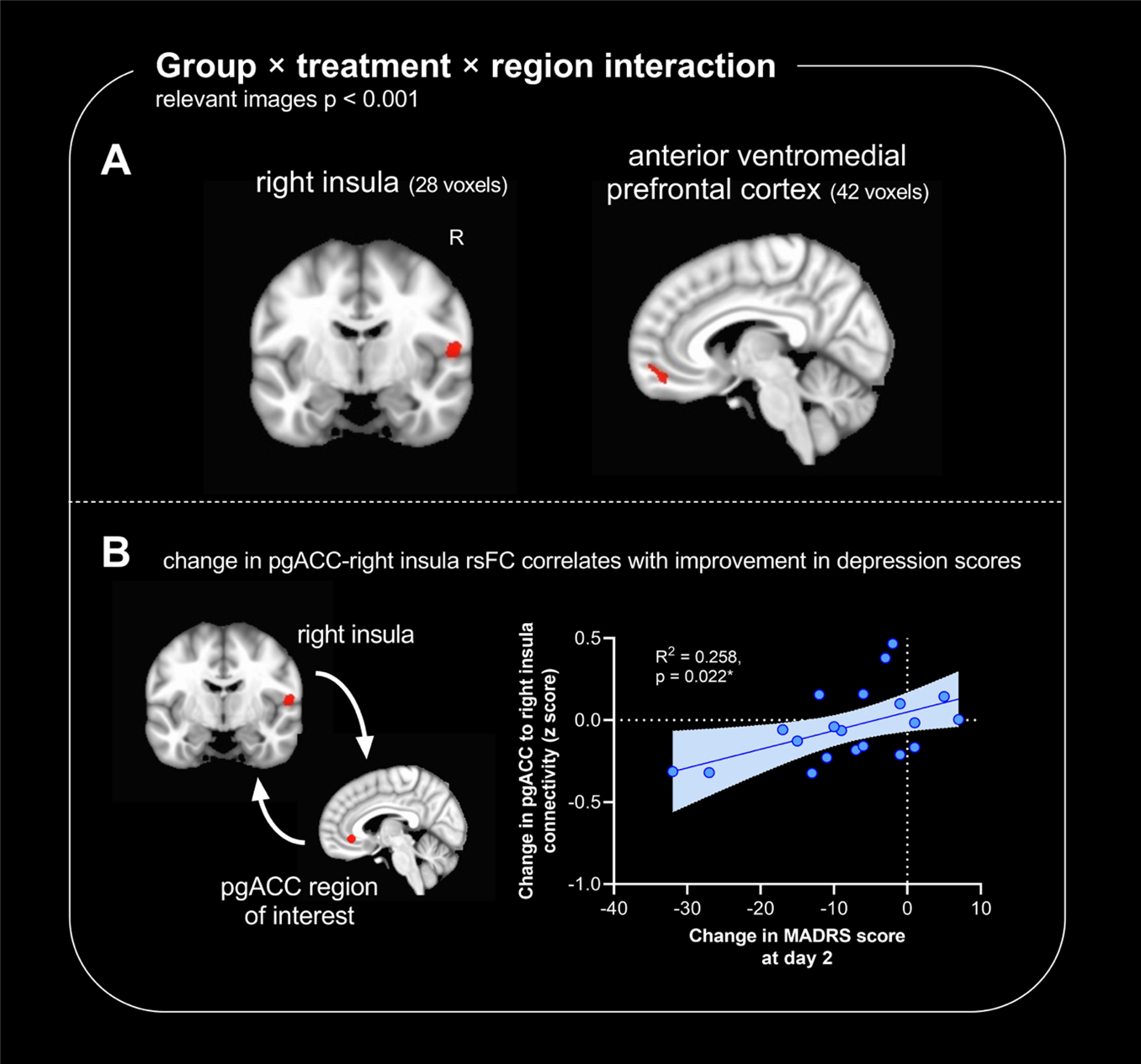
In the clinic, patients are only compared to themselves, so we then specifically explored the effects of ketamine vs. placebo on brain connectivity in people with TRD. When we did this, the ACC region most substantially modulated was the sgACC. Namely, rsFC of sgACC to the pgACC, the anterior ventromedial prefrontal cortex, the right ventral striatum, and the right hippocampal and parahippocampal gyrus was significantly altered. Whilst these changes did not correlate with improvements in depression scores, changes to sgACC-pgACC and sgACC-ventral striatal rsFC correlated with improvements in anhedonia scores. This suggests that sgACC is an important part of a reward-related network which is modulated by ketamine to alleviate problems in reward processing seen in depression. This is supported by preclinical studies in animals, which highlight that ketamine modulates a reward-related brain network to alleviate deficits in reward processing8,9.
What are the strengths and limitations of our work?
These data are the first direct comparison of ketamine’s modulation of different ACC subregions within a single study, carried out in the context of a double-blind randomized controlled crossover trial. The trial was well designed: blinding minimizes observer bias; the crossover design minimizes the effects of confounding factors between individuals and increases statistical power; and there was a reasonable washout period (of two weeks) between placebo and ketamine doses. Whilst imaging data are by their nature correlative, they back-translate to preclinical animal models in both primates and rodents which suggest that ACC modulations are causally involved in ketamine’s antidepressant and anti-anhedonic effects3.
It is important to recognize the limitations of this work. First, whilst mitigated by the crossover design, the sample size was small, especially when exploring the correlations between rsFC changes and symptom scores. Second, a fixed time-point (two days post ketamine or post placebo) precluded an exploration of how ketamine-induced rsFC changes develop or change over time. Third, whilst the trial was double-blinded and placebo controlled, there is the possibility of unblinding due to the subjective effects of ketamine compared to an inactive placebo.
How does this work highlight the benefits of open science?
The work came about from a collaboration between researchers at KCL and the NIMH. The corresponding author had a hypothesis to test, and it was through the sharing of expertise, resources, data, and perspectives that the study was completed and an outcome was achieved that would not have been possible otherwise. Collaboration in science fosters innovation, creativity, diversity, and quality, and benefits both the scientific community and society at large. This project is but one example of how cross-institutional collaboration in science can lead to important discoveries that shape the direction of future research and our understanding of the neurobiology of how psychiatric treatments work.
References
1 Abdallah C.G., Averill L.A., Krystal J.H. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann New York Acad Sci 2015; 1344: 66–77.
2 Wilkowska A, Wiglusz MS, Gałuszko-Wegielnik M, Włodarczyk A, Cubała WJ. Antianhedonic Effect of Repeated Ketamine Infusions in Patients With Treatment Resistant Depression. Frontiers in Psychiatry 2021; 12.https://www.frontiersin.org/articles/10.3389/fpsyt.2021.704330 (accessed 19 Apr2023).
3 Alexander L, Jelen LA, Mehta MA, Young AH. The anterior cingulate cortex as a key locus of ketamine’s antidepressant action. Neuroscience & Biobehavioral Reviews 2021; 127: 531–554.
4 Brodmann K. Localisation in the Cerebral Cortex. 1909.
5 Evans JW, Szczepanik J, Brutsché N, Park LT, Nugent AC, Zarate CA. Default Mode Connectivity in Major Depressive Disorder Measured Up to 10 Days After Ketamine Administration. Biological Psychiatry 2018; 84: 582–590.
6 Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE et al. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Molecular Psychiatry 2019; 24: 1040–1052.
7 National Institute of Mental Health (NIMH). Investigation of the Rapid (Next Day) Antidepressant Effects of an NMDA Antagonist. clinicaltrials.gov, 2018https://clinicaltrials.gov/study/NCT00088699 (accessed 1 Jan2023).
8 Alexander L, Gaskin PLR, Sawiak SJ, Fryer TD, Hong YT, Cockcroft GJ et al. Fractionating Blunted Reward Processing Characteristic of Anhedonia by Over-Activating Primate Subgenual Anterior Cingulate Cortex. Neuron 2019; 101: 307-320.e6.
9 Wood CM, Alexander L, Alsiö J, Santangelo AM, McIver L, Cockcroft GJ et al. Chemogenetics identifies separate area 25 brain circuits involved in anhedonia and anxiety in marmosets. Science translational medicine 2023; 15. doi:10.1126/scitranslmed.ade1779.
10 Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Feb 28, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in