Lewis acid-catalyzed asymmetric reactions of β,γ-unsaturated 2-acyl imidazoles
Published in Chemistry

In 2019, we wondered if a well-designed olefin substrate could underwent a radical addition reaction in the presence of N,Nʹ-dioxide-based chiral Lewis acid catalysts. After the preliminary investigation of several α,β-conjugated electron-deficient alkenes, regrettably, no desired radical addition products. We then devoted ourselves to the modification of substrates and a series of deconjugated alkenes were examined. When the substrate with functional 1,3-dicarbonyl groups as the directing group was used, there was still no reaction. What fascinating us is racemic product can be obtained when 1,3-N,O-chelating functional group was installed into the olefin. β,γ-Unsaturated 2-acyl imidazole could give the desired product with good enantioselectivity along with the formation of an unknown compound as the major product, which was determined to be a self-dimerization product of β,γ-unsaturated 2-acyl imidazole, and moderate diastereoselectivity and excellent enantioselectivity were obtained. Intrigued by this unexpected transformation, some mechanistic studies and control experiments were performed. The results exhibited that the self-dimerization product could also be formed without radical reaction conditions, which suggested that the reaction underwent a Lewis acid-catalyzed ionic transformation. The succulently meticulous screen of the reaction conditions provided a promising result, as described in the manuscript, the reaction of β,γ-unsaturated 2-acyl imidazole occurred to afford the self-dimerization products only in the presence of chiral N,Nʹ-dioxide-lanthanide complexes.
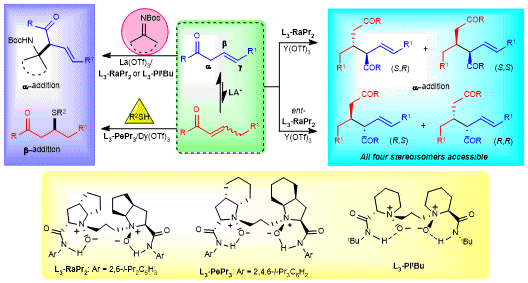
During the screening of the reaction conditions, only moderate diastereoselectivity was observed after variety of metal salts, chiral ligands and solvents were tested. Delightedly, the dr value could be improved with addition of NEt3 and intrigued us to explore how did NEt3 work. It was found that the diastereoselectivity was determined by the configuration of the α,β-unsaturated 2-acyl imidazole intermediate, which was identified by a series of control experiments. (E)-α,β-Unsaturated 2-acyl imidazole gave the anti-product, and (Z)-α,β-Unsaturated 2-acyl imidazole gave the syn-product. The role of NEt3 was to increase the ratio between (E)- and (Z)-α,β-unsaturated 2-acyl imidazole, thus resulting in higher diastereoselectivity.
Another beauty of this work is that the designed β,γ-unsaturated 2-acyl imidazoles could not only serve as nucleophiles but also electrophiles, which was rarely reported in the former literatures1,2. A number of aldimines and ketimines were tolerated in the direct α-Mannich reaction, delivering functionalized β-amino ketones with excellent diastereo- and enantioselectivities. The tandem isomerization/sulfur Michael addition also indicated that the β,γ-unsaturated 2-acyl imidazoles could also play a role as electrophiles which will inspire chemists to study the chemistry of β,γ-deconjugated carbonyl compounds.
References:
1. Mitsudo, N. Suzuki, T. Kondo, Y. Ruthenium complex-catalyzed carbonylation of allylic compounds. J. Org. Chem. 59, 7759–7765 (1994).
2. Olaizola, I. Iriarte, G. Zanella, E. Gómez-Bengoa, I. Ganboa, M. Oiarbide, C. Palomo. Brønsted base catalyzed one‐pot synthesis of stereodefined six‐member carbocycles featuring transient trienolates and a key intramolecular 1,6‐addition. Angew. Chem. Int. Ed. 58, 14250–14254 (2019).
You can read more about our work in our Nature Communications paper: https://www.nature.com/articles/s41467-020-17681-9.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in