Light modulates task-dependent thalamo-cortical connectivity during an auditory attentional task
Published in Neuroscience

Light provides more than just visual information through non-classical photoreception (also referred to as Non-Image Forming – NIF effects)1–3. Non-visual responses affect physiological processes such as circadian entrainment, heart rate, body temperature, hormone secretion, pupil light reflex, alertness, and sleep propensity. These responses are mediated to a large extent by intrinsically photosensitive retinal ganglion cells (ipRGCs) that constitute a distinct class of photoreceptors expressing the photopigment melanopsin, most sensitive to blue wavelength light at around 480nm4,5.
Light has a direct activating effect on cognition and acutely increases performance following the onset of light1,2,6. However, the neural processes underlying light-induced modulation of non-visual cognitive performance are not fully established. The pulvinar, posterior thalamic nuclei, is one of the structures most consistently activated in response to light in human fMRI studies of the impact of light on ongoing non-visual cognitive activity6–9. It is involved in attention control and, together with adjacent multimodal associative nuclei, modulates ongoing cortical activity through recurrent thalamocortical loops10,11. Importantly, changes in thalamic activity were found to be directly related to the subjective improvement of alertness induced by light exposure9. The posterior thalamus could therefore be an essential structure through which light affects the information flow in the brain for the completion of non-visual cognitive tasks.
In our article “Light modulates task-dependent thalamocortical connectivity during an auditory attentional task” recently published by Communications Biology, we provide empirical support for this assumption. In an ultra-high-field 7 Tesla MRI scanner, we recorded the brain activity of 19 healthy young participants while they completed a purely auditory attentional task known to recruit the posterior thalamus. Participants had to detect rare deviant tones presented pseudo-randomly within a stream of more frequent standard ones. While performing the task, they were alternatively maintained in darkness or exposed to active, blue-enriched polychromatic, or control orange monochromatic light, which, importantly, differ in terms of their predicted stimulation of ipRGCs. During the fMRI session participants’ pupil size was also recorded.
In line with prior literature, a widespread set of brain regions were shown to respond to deviant tones, including a bilateral activation of the anterior intraparietal sulcus (IPS) and the dorso-posterior thalamus, which are implicated in top-down regulation of attention12 and target detection13 respectively. Using Dynamic Causal Modelling (DCM)14, we investigated the baseline effective connectivity between these two key brain areas for attentional control and if and how light information exerted a modulation on the connectivity. At baseline, we saw a reciprocal negative influence between both regions, on top of a relatively weak self-inhibitory feedback for the left thalamus and a relatively stronger self-inhibition feedback bilaterally for IPS (Figure 1a). Critically, when considering the modulatory effect of the active (Figure 1b) and the control (Figure 1c) lights, the analyses yielded a single significant modulation of the connection going from the posterior thalamus to the IPS connection only under the active, blue-enriched light condition which switched the influence of the posterior thalamus on IPS from inhibition to excitation. On a subset of 11 participants (only those with good pupil data) we further found that this increased thalamus-to-IPS effective connectivity under blue-enriched light was negatively correlated with the difference in pupil constriction in both light exposures (Figure 1d).
To summarize, in line with our hypothesis, we found (1) that the connection from the thalamus to the IPS was selectively modulated by light and (2) that only blue-enriched light exerted a significant modulation of the information flow along this connection. In other words, the active light modulated specifically the information flow from thalamic to cortical areas, while the control light, despite otherwise eliciting visual responses, was not affecting our network in any way. Non-visual light information is transferred to the brain through the optic nerve by ipRGCs, which seem to constitute the only channel through which light triggers non-visual responses15. A single ipRGC can target up to five different brain areas involved in many distinct light-mediated behaviors. There are several pathways light could use to reach the thalamus. Based on our data, we cannot, however, isolate which pathway may be involved in the effects of light we detected. The photic sensory input could first reach the lateral geniculate nucleus (LGN) and then the primary visual cortex. This primary sensory area could then send the information back to both the thalamic relay nucleus and to a high order-association nuclei, such as the pulvinar, which could in turn drive alertness in associative cortical regions16 (e.g., parietal cortex and IPS specifically). Despite being an exploratory analysis on a subset of 11 participants, the fact that the thalamus-to-IPS modulation exerted by blue-enriched light was correlated to the changes in pupil constriction induced by blue-enriched light, may support that our connectivity findings consist of a non-visual response to light. Pupil response to light constitutes indeed a distinct NIF response mostly not relying on the pulvinar and IPS. It could therefore be considered as an independent assessment of the NIF impact of light that is not dependent on the visual pathway. Critically, the fact that the exposure to the active light shifts the thalamocortical connection from inhibitory to excitatory could enhance neural communication by making it more effective, precise, and selective, eventually resulting in a “wake-up call” to widespread cortical territories involved in the ongoing cognitive processes17.
To conclude, we provided an original empirical demonstration that light affects non-visual ongoing cognitive processes at least through the modulation of the thalamocortical information flow in the brain. This is the first step to unravel the connectivity changes taking place during (blue) light exposure therefore clarifying the pathway linking the retina to cognition.
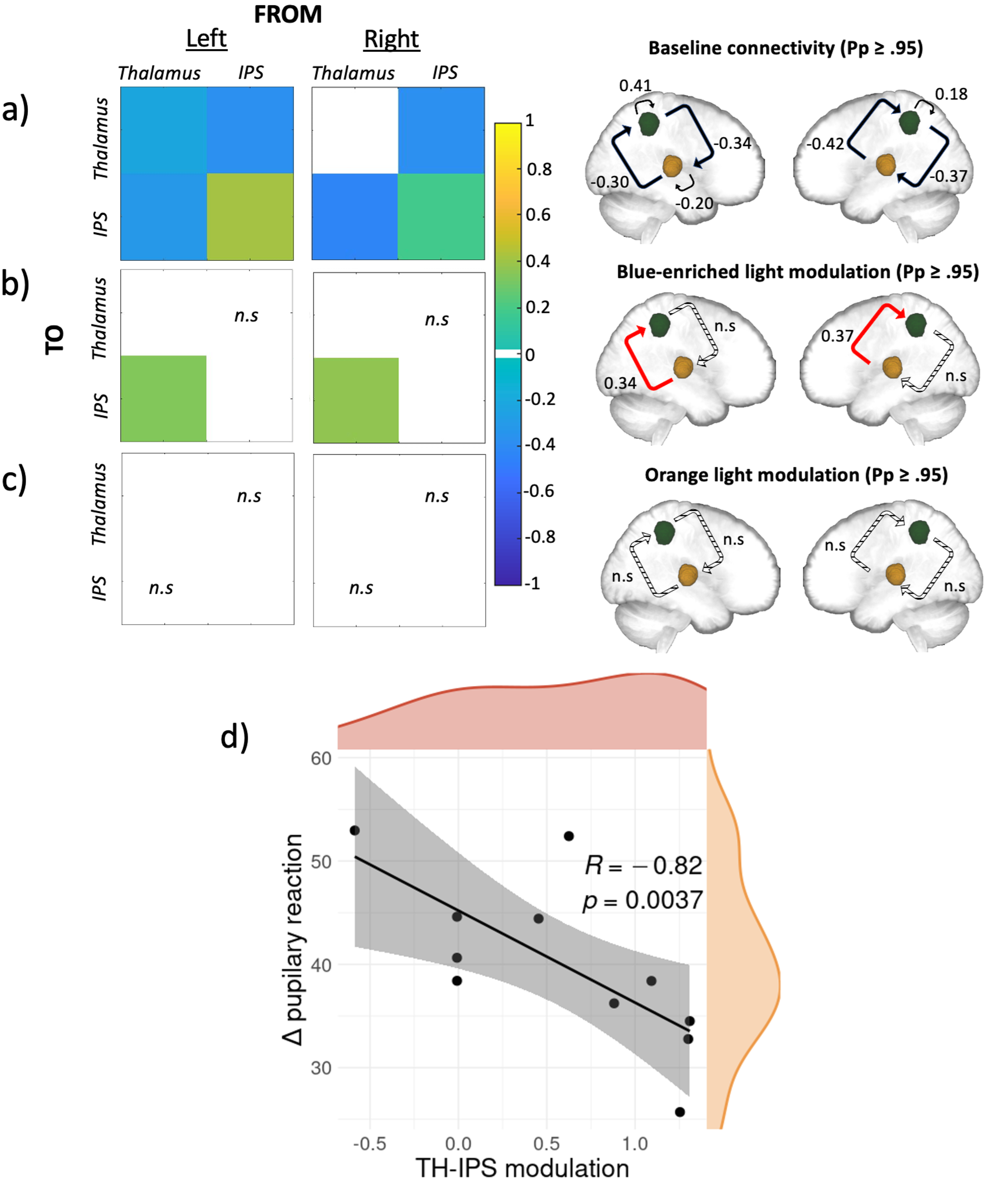
Figure 1. Effective connectivity results and their relationship with pupillary response. a, b, and c) Results of the DCM analysis at baseline, under blue-enriched or orange light respectively. Left: matrices of the effective connectivity either at baseline (a) or with modulatory effects exerted by the active blue-enriched (b) or the control orange (c) light for either the left or the right hemisphere. Right: schematic representation of the corresponding matrices where IPS and the thalamus are shown in green and yellow respectively. On all panels, only suprathreshold parameters are shown (Pp > 0.95) whereas subthreshold parameters are marked as “n.s” (i.e., non-suprathreshold). Connections strengths are represented on a scale from turquoise to yellow, if excitatory, and from light to dark blue if inhibitory. Non modelled direct effects (i.e., whose priors were set to 0) are displayed in white. In the schematic representation, the line patterns denote whether the connection was significantly modulated compared to baseline: solid and dashed lines represent connections that were significantly modulated or not, respectively; red lines denote excitatory connections whereas black ones denote inhibitory connections. d) Spearman correlation results. Spearman correlation between modulation exerted by blue-enriched light over the thalamus-to-IPS connection (referenced to baseline) across both hemispheres and the difference in pupil constriction in both lights exposures (r[9]= -0.82; p = 0.0037). Density plots for both variables are also provided in orange and red respectively.
References
- 1. Brown, T. M. et al. Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLoS Biol. 20, e3001571 (2022).
- Fisk, A. S. et al. Light and cognition: roles for circadian rhythms, sleep, and arousal. Front. Neurol. 9, 56 (2018).
- Campbell, I., Sharifpour, R. & Vandewalle, G. Light as a Modulator of Non-Image-Forming Brain Functions—Positive and Negative Impacts of Increasing Light Availability. Clocks & Sleep 5, 116–140 (2023).
- Berson, D. M., Dunn, F. A. & Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science (80-. ). 295, 1070–1073 (2002).
- Schmidt, T. M., Chen, S.-K. & Hattar, S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 34, 572–580 (2011).
- Gaggioni, G., Maquet, P., Schmidt, C., Dijk, D.-J. & Vandewalle, G. Neuroimaging, cognition, light and circadian rhythms. Front. Syst. Neurosci. 8, 126 (2014).
- Vandewalle, G. et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS One 2, e1247 (2007).
- Vandewalle, G. et al. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb. cortex 17, 2788–2795 (2007).
- Vandewalle, G. et al. Daytime light exposure dynamically enhances brain responses. Curr. Biol. 16, 1616–1621 (2006).
- Saalmann, Y. B., Pinsk, M. A., Wang, L., Li, X. & Kastner, S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science (80-. ). 337, 753–756 (2012).
- Guillery, R. W. & Sherman, S. M. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron 33, 163–175 (2002).
- Uncapher, M. R., Hutchinson, J. B. & Wagner, A. D. Dissociable effects of top-down and bottom-up attention during episodic encoding. J. Neurosci. 31, 12613–12628 (2011).
- Ardekani, B. A. et al. Functional magnetic resonance imaging of brain activity in the visual oddball task. Cogn. Brain Res. 14, 347–356 (2002).
- Friston, K. J., Harrison, L. & Penny, W. Dynamic causal modelling. Neuroimage 19, 1273–1302 (2003).
- Güler, A. D. et al. Melanopsin cells are the principal conduits for rod–cone input to non-image-forming vision. Nature 453, 102–105 (2008).
- Saalmann, Y. B. & Kastner, S. Gain control in the visual thalamus during perception and cognition. Curr. Opin. Neurobiol. 19, 408–414 (2009).
- Sherman, S. M. & Guillery, R. W. Exploring the thalamus and its role in cortical function. (MIT press, 2006).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in