Lipid Nanomachines Overcome Endo−Lysosomal Barrier
Published in Biomedical Research
How did we come up with the idea?
Nanoparticles have become crucial vehicles for delivering biologic drugs into cells. During the intracellular transport, it is essential for the biologics to overcome the endo−lysosomal barrier for successful intracellular delivery. For example, the mRNA COVID-19 vaccines rely on the lipid nanoparticles (LNP) to deliver exogenous mRNA into the cells and express antigens for a boosted immune response against the virus. As a key component of LNP, an ionizable lipid is designed to disrupt the endosomal membrane in response to the acidic pH and facilitating the efficient release of mRNA into the cytoplasm. Identifying a suitable ionizable lipid involves screening numerous candidates, which is a time-consuming and laborious process. Moreover, specific ionizable lipids are tailored to deliver particular cargoes to specific cell types. To tackle this challenge, we came up with an idea of designing a universal lipid capable of overcoming the endo−lysosomal barrier, regardless the cargo it carries.
Our approach
We designed an azobenzene (Azo)-based lipid that can convert light energy into mechanical movements (Fig. 1). Doping this Azo-based lipid into the LNP, a lipid nanomachine (LNM) is formed to realize our hypothesized function. Specifically, the Azo-based lipids in LNMs are in a reversible form of compact cis- and extended trans-isomers in response to the irradiation with ultraviolet (UV, 365 nm) and visible light (Vis, >400 nm), respectively. In the presence of simultaneous UV and Vis (UV/Vis) irradiation, the Azo-based lipids in LNMs undergo continuous rotation−inversion and stretch−shrink movements. As a result, after the LNMs enter the cells via endocytosis, the Azo-based lipids act as rotors continuously destabilizing the endo-lysosomal membrane and enhancing the release of the cargos.
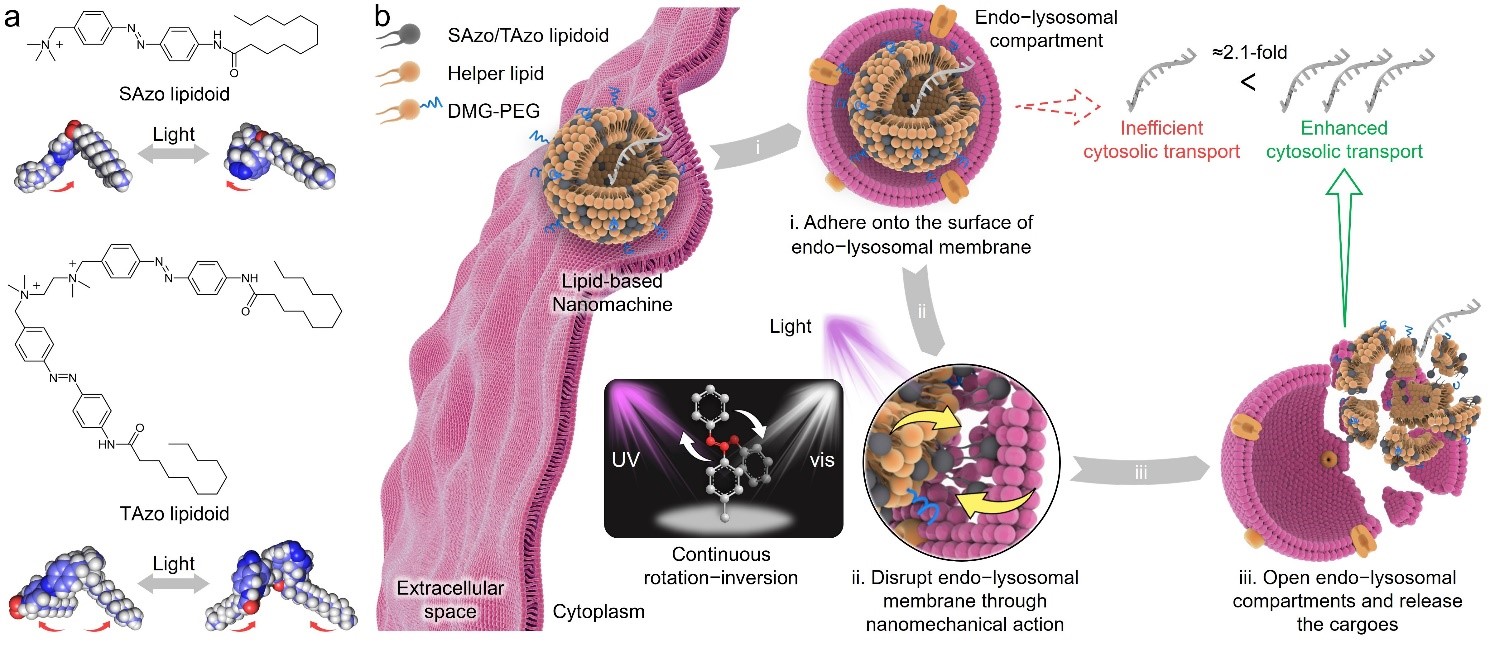
Fig. 1 Lipid-based nanomachine (LNM) opens endo−lysosomal compartments through nanomechanical action. a) Chemical structures of the two different Azo-based lipidoids, including SAzo lipidoid and TAzo lipidoid. b) Schematic illustration of the LNM structure and its potential mechanism of overcoming intracellular barriers through light-triggered nanomechanical action.
Our key findings
- We identified an Azo-base lipid “TAzo” showing the best endo−lysosomal escape efficiency.
- We found that the transport of both nucleic acid (e.g., mRNA) and protein (e.g., Cre protein) to the cytoplasm could be facilitated by our LNM. which provides a solution to improve the intracellular delivery of therapeutic biologics.
- We further investigated LNM-mediated antigen cross-presentation in murine bone marrow-derived dendritic cells (BMDCs). Adopting ovalbumin (OVA) as a model antigen, BMDCs treated with TAzo-LNM/OVA complexes exhibited 2.6-fold higher presentation of OVA fragments after UV/Vis light irradiation, compared to the group without light irradiation. This engineered DCs induced robust cytotoxic T cell activation and antitumour immune memory. In the rechallenging study, the treatment with TAzo-LNM/OVA + UV/Vis significantly inhibited tumour metastasis to the lungs.
Team behind the paper
"In the process of selecting the most suitable ionizable lipid for nucleic acid delivery, a bottleneck has persisted despite extensive screening efforts. Researchers in my lab previously tested hundreds of ionizable lipids, but few proved effective, and their delivery efficiency varied across different cell types," explained Dr. Qiaobing Xu, a Professor at Tufts University who supervised this study. "Building a new biologic delivery system that bypasses the endo-lysosomal barrier and is compatible with various cell types is truly exciting."
"Our research opens a promising avenue for creating man-made molecular machines with biological functions through straightforward chemical processes. This approach holds significant potential in developing soft robotics and intelligent drug delivery platforms," stated Dr. Yu Zhao, a postdoctoral researcher at Tufts University and the paper's lead author. Dr. Zhao made substantial contributions to synthesizing Azo-based lipids, assessing the efficiency of LNM-mediated endo-lysosomal escape, and exploring the application of LNMs in DC-based cancer immunotherapy.
"Our 'nanorobots' share a similar structure and preparation procedure with clinically used LNPs, making this strategy applicable to a wide range of therapeutic nucleic acids and proteins," said Dr. Donghui Song, another postdoctoral researcher at Tufts University. Dr. Song significantly contributed to identifying the optimal formulation of LNMs by screening excipient compositions and ensuring the stability of cargo carried in LNMs during UV/Vis light irradiation. His work lays the groundwork for applying LNMs in cancer treatment through DC-based immunotherapy.
The study is entitled “Nanomechanical action opens endo-lysosomal compartments” published in npj Nature Communications, 14, 6645 (2023)" Link is below:
Nanomechanical action opens endo-lysosomal compartments | Nature Communications
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in