Lithium‑Ion Dynamic Interface Engineering of Nano‑Charged Composite Polymer Electrolytes for Solid‑State Lithium‑Metal Batteries
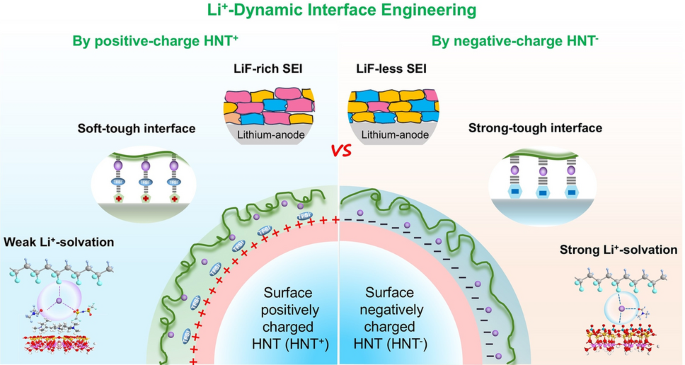
Solid-state lithium-metal batteries (SSLMBs) are the holy-grail of next-generation energy storage, but their commercialization has been stymied by dendrite growth, fragile interfaces, and the ion-conductivity vs. mechanical-strength trade-off. Now, researchers from Sichuan University, led by Prof. Yu Wang and Prof. Xuewei Fu, have introduced a “lithium-ion dynamic interface (Li+-DI)” strategy that turns charged halloysite nanotubes (HNTs) into nano-interfacial engineers, delivering composite polymer electrolytes (NCCPEs) that are simultaneously super-tough, highly conductive, and dendrite-suppressing.
Why Surface Charge Engineering Matters
-
Breaks the Toughness–Conductivity Trade-off:
Positively charged HNT+ creates a soft-and-tough Li+-DI, boosting toughness by >2000 % while maintaining 0.19 mS cm-1 ionic conductivity and a record-high Li+ transference number (0.86). -
LiF-Rich SEI on Demand:
HNT+ lowers the LUMO of TFSI-, steering its preferential decomposition into a LiF-rich, mechanically robust SEI that suppresses dendrites and enables 700 h of symmetric-cell cycling at 0.2 mA cm-2. -
Universal Cathode Compatibility:
Li|NCCPE|LFP retains 78.6 % capacity after 400 cycles (0.5 C); Li|NCCPE|NCM811 delivers 74.4 % retention after 200 cycles at 4.4 V—outperforming most reported PVDF-based electrolytes.
Key Innovations
-
Charged 1D Nanofillers:
Electrostatic self-assembly of PDDA (HNT+) or hexametaphosphate (HNT-) tailors zeta potential (+46 vs –43 mV), eliminating nanotube agglomeration and creating percolated ion highways inside 40 µm-thin membranes. -
Dynamic Li+ Bridge:
DFT and TS-DFT reveal that HNT+ anchors TFSI-, forcing Li+ to hop through an anion-rich, solvent-assisted pathway with 0.69 eV barrier—35 % lower than uncharged interfaces. -
Scalable Solution Processing:
Doctor-blading + vacuum drying yields binder-free, flexible films compatible with roll-to-roll fabrication and existing Li-ion infrastructure.
Mechanistic Insights
-
Anion-Rich Solvation Sheath:
Raman + ss-NMR show HNT+ promotes CIP/AGG species (57 %) vs HNT- (41 %), weakening Li+–solvent coordination and widening the electrochemical window to 4.8 V. -
Dendrite-Free Li Plating:
SEM/XPS confirm smooth, dense Li deposits with >91 % Coulombic efficiency and 2× higher LiF content—no dead Li or dendrites even at 1 mA cm-2. -
Inner-Tube Nano-Confinement:
1D HNT lumen acts as a DMF reservoir, plasticizing the interface and relieving stress during volume expansion, extending cycle life under practical areal loadings (3.5–4 mAh cm-2).
Future Outlook
-
Next-Gen SSLMBs:
The Li+-DI concept is material-agnostic—transferable to LLZO, MOF, or polymer fibers—offering a universal toolbox for solid-state Na, Zn, and multivalent batteries. -
Fast Commercialization:
With low-cost halloysite, eco-friendly processing, and record performance, NCCPEs are poised to bridge the lab-to-market gap for safe, energy-dense EV and grid-storage packs. -
AI-Driven Optimization:
Machine-learning integration of surface-charge descriptors could accelerate the discovery of next-wave nanofillers and push energy densities beyond 400 Wh kg-1.
This work establishes surface-charge engineering as a paradigm shift in composite electrolyte design, transforming inert nanofillers into active interfacial architects for dendrite-free, long-life solid-state batteries.
Stay tuned for more breakthroughs from Prof. Yu Wang and the Sichuan University team!
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in