Longitudinal human epidermal and dermal transcriptomic and spatial gene profiling at the incisional edge during long surgical procedures
Published in Cell & Molecular Biology, Biomedical Research, and Pharmacy & Pharmacology
Surgery, by its very nature, is an injury. It provides a rare window into how human skin responds at the molecular level to a noxious stimulus, offering insights into the signals that contribute to pain. Our research team from the National Institutes of Health has published an article in Communications Biology, “Longitudinal human epidermal and dermal transcriptomic and spatial gene profiling at the incisional edge during long surgical procedures.” This study investigates what Dr. Michael Iadarola, a senior scientist on the project, called “how pain begins.”
As Dr. Andrew Mannes put it, this unique human injury model follows the same patient over the course of surgery, examining within-subjects alterations in gene expression over time.
The central aim of our work has been to leverage transcriptomics to understand the mechanisms of pain and pain control, with the broader goal of developing novel non-opioid analgesics, such as the lead compound resiniferatoxin (RTX).
One of the core questions that our laboratory has grappled with is the translatability from preclinical rodent models to human pathology. In previous studies, we had observed marked differences in gene expression of the kappa opioid receptor, for example, between mice, rats, dogs, and humans—underscoring the importance of validating mechanistic hypotheses directly in human tissue.
In a previous study led by Stephen Raithel1, our laboratory evaluated the analgesic potential of the novel non-opioid analgesic and TRPV1 agonist resiniferatoxin (RTX) in a rat model of post-surgical pain. Post-surgical pain is of particular interest to us because it commonly affects opioid-naïve patients and can be difficult to treat, sometimes leading to persistent neuropathic pain. The motivation to identify new non-opioid options for managing post-surgical neuropathic pain helped inspire the human-based investigation reported in our new publication.
Building on two earlier studies led by Taichi Goto2,3, which examined longitudinal transcriptomic signatures in rodent incision and inflammation models, our newly published work sought to extend these insights into humans. We analyzed tissue specimens collected during long surgical procedures using bulk transcriptomics, lipidomics, and spatial mRNA mapping via RNAscope, enabling a multi-layered "multi-omics" view of molecular changes at the incision edge.
A critical component of this project was the unique clinical tissue collection protocol we developed as a team. Biopsies were obtained directly in the operating room, at precise time intervals, with surgeons handing samples immediately to investigators (Drs. Domenichiello and Sapio) for rapid processing. This “hands-on” workflow ensured precise tissue handling and preservation, with a fine attention to small details (including knowing which edge of tissue specimen was the initial incision edge). We believe this "high touch" approach was essential to the study’s success. These methods are shared in an associated published protocol (10.17504/protocols.io.j8nlk81w1l5r/v1).
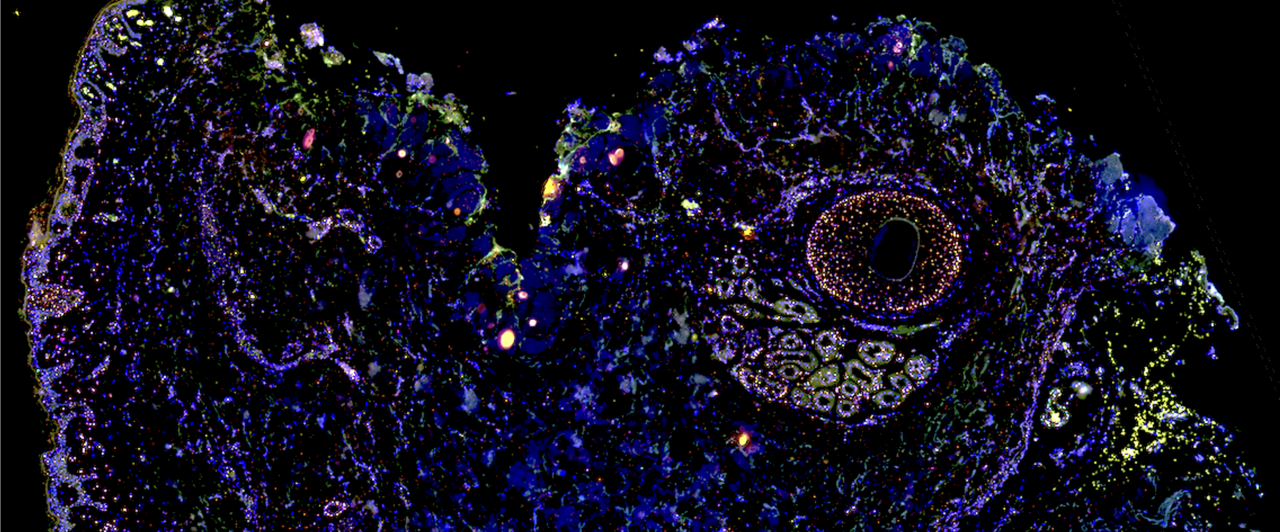
We also developed a photobleaching device to reduce autofluorescence from human fixed formalin paraffin embedded tissue sections, which was critical to obtaining usable multiplex fluorescence microscopy data4.
Our findings revealed rapid and strong induction of secreted signaling molecules, including interleukin 6 (IL6), interleukin 8 (IL8), metallothionein 2A (MT2A), oncostatin M (OSM) with remarkably low inter-patient variance despite differences in age, sex, and surgical indication.
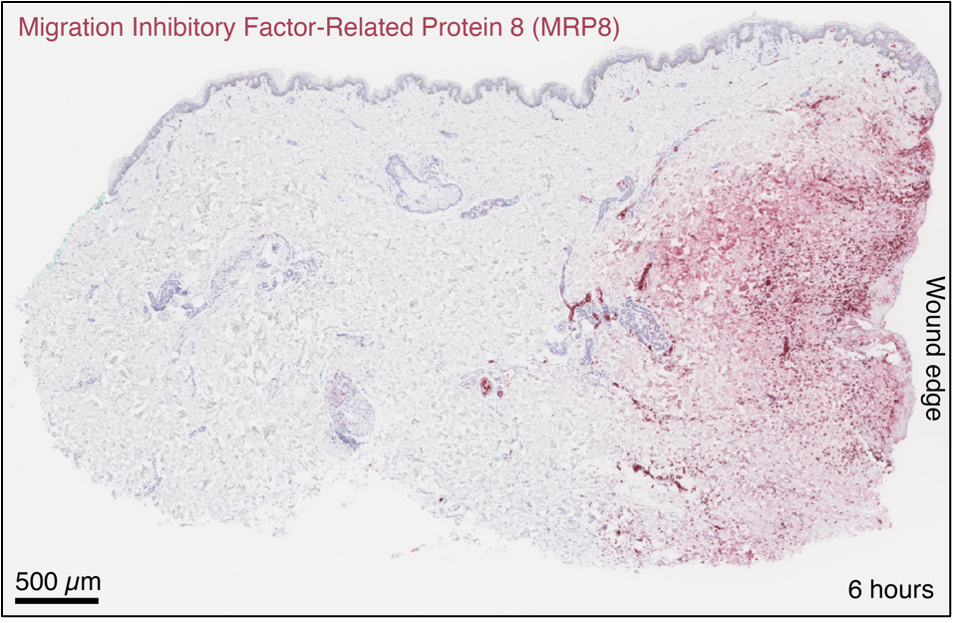
The neutrophil marker MRP8 accumulates at the wound edge.
In situ hybridization localized the transcripts primarily to deeper follicular structures and gland tissues, particularly around perifollicular sebaceous glands and eccrine sweat glands,—sites of higher cellular density relative to the sparsely populated dermal interstitium.
We further analyzed our transcriptomic data to assess the ability of these molecules to signal to dorsal root ganglion neurons as part of the peripheral sensitization pathway occurring with surgery. Ultimately, we posited that the network of secreted factors that we called “the nociceptive secretome” informs analgesic development. We are now working in a collaborative fashion to better understand the algogenic and analgesic potential of signaling molecules induced by incisional injury. In these “back translation” studies, we hope to better understand the mechanistic implications of the induced signaling pathways that occur after incision injury in humans.
In aggregate, we hope that this work provides a molecular framework for understanding wound responses in human skin and can be useful for illuminating future studies in the larger drug discovery effort.
Acknowledgements
This post was written by Dr. Matthew R. Sapio. Critical contributions were made by Evelyn Li, who completed the in situ hybridization staining and imaging work in this study, and was an integral part of its completion, as well as Dr. Anthony F. Domenichiello (pictured). We also acknowledge Ofek Blivis and Michael G. Dunkelberg for artistic commentary.
Funding and Disclaimer
This work was funded by the National institutes of Health, Clinical Center, and refers to work funded by the National Institutes of Health, National Cancer Institute, National Institute of Neurological Disorders and Stroke, among other funding sources (1ZIACL090033, 1ZIACL090034, and 1ZIACL090035 to AJM, see primary article). The contributions of the NIH author(s) are considered Works of the United States Government. The findings and conclusions presented in this paper are those of the author(s) and do not necessarily reflect the views of the NIH or the U.S. Department of Health and Human Services.
References
1. Raithel SJ, Sapio MR, LaPaglia DM, Iadarola MJ, Mannes AJ. Transcriptional Changes in Dorsal Spinal Cord Persist after Surgical Incision Despite Preemptive Analgesia with Peripheral Resiniferatoxin. Anesthesiology 128(3):p 620-635, 2018. DOI: 10.1097/ALN.0000000000002006
2. Goto T, Sapio MR, Maric D, Robinson JM, Saligan LN, Mannes AJ, Iadarola MJ. Longitudinal Transcriptomic Profiling in Carrageenan-Induced Rat Hind Paw Peripheral Inflammation and Hyperalgesia Reveals Progressive Recruitment of Innate Immune System Components. J Pain 22(3):p322-343, 2021 https://doi.org/10.1016/j.jpain.2020.11.001
3. Goto T, Sapio MR, Maric D, Robinson JM, Domenichiello AF, Saligan LN, Mannes AJ, Iadarola MJ. Longitudinal peripheral tissue RNA-Seq transcriptomic profiling, hyperalgesia, and wound healing in the rat plantar surgical incision model. FASEBJ 2021. 35:e21852. https://doi.org/10.1096/fj.202100347R
4. Sapio MR, King DM, Maric D, Shah SR, Talbot TL, Manalo AP, Nara P, Ma W, Ghetti A, Ramsden CE, Iadarola MJ, Mannes AJ. Efficient removal of naturally-occurring lipofuscin autofluorescence in human nervous tissue using high-intensity white light. J Pain 30. 2024. https://doi.org/10.1016/j.jpain.2025.105359
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in