Losing the larval stage… one step at a time?
Published in Ecology & Evolution
Amphibians do some pretty spectacular things. Something that had always interested me is the shear diversity in places where you can find eggs and tadpoles. Those of us who have spent any time at all looking for frogs in the tropics will know that it is not uncommon to find eggs stuck to vegetation or encounter larvae in water filled tree holes. Even in more temperate latitudes, at the right time of year, male midwife toads can be seen caring eggs stuck to their hind limbs. Only once tadpoles are ready to hatch do they take them to water where they continue their development.

Why should we be interested in any of this? Well, despite what our anthropocentric perspective may lead us to think, the majority of animals have complex life cycles, consisting of one or more larval phases, followed by the reproductive adult phase. These phases are typically very distinct both in their morphology and ecology. The transition in phases therefore involves extensive re-modelling of body plans, a process called metamorphosis. A minority of animals however (including us mammals), do not have such complex life cycles and instead produce offspring that already largely resemble their reproductive adults. How does such a major evolutionary shift in life cycle occur? How can organisms go from having complex life cycles to simple ones? These must surely be major moments in the evolutionary history of lineages.
To study this question in vertebrates, amphibians make for a great system. Complex life cycles are common and quite extreme (think of tadpoles transforming into frogs!). However, not all amphibians have larval stages. All three major groups of amphibians, the frogs (Anura), salamanders (Caudata) and caecilians (Gymnophiona) have repeatedly lost the larval stage, with lineages evolving from the ancestral complex life cycles with multiple life stages to a simple life cycle with a single life stage. Moreover, as I mentioned earlier, both complex and simple life stages in all three groups come in many different flavours. In fact, amphibians are often quoted as being the group of tetrapods with the most diversity in “Reproductive modes”. These modes diverge primarily in whether or not species lay eggs or give birth to live young (oviparity versus viviparity), whether a free-living larval stage exists or not (direct development) or whether larva and eggs develop in water or on land (aquatic versus terrestrial). Beyond that, more fine-scaled divisions can be made as to what substrate eggs are attached to or whether larva actively feed or develop only using reserves of energy (exotrophy versus endodrophy).
Now that I have convinced you that amphibians are a great system, how do we go about testing what the most likely evolutionary scenario was for transitions in reproductive modes? With just a phylogenetic tree and traits mapped to each species in the tree (in our case categorical reproductive modes), we can use comparative phylogenetic methods to estimate the rates at which traits have shifted from one state to another across the tree. We can also see whether we end up with a better fitting model if we rule out certain state shifts completely. By only permitting specific transitions, we can start to build hypotheses on how we thing reproductive modes evolve. For example, we could pit a scenario where all transitions are possible (a “Null” scenario) against scenarios where all derived states evolve directly from the most ancestral state, or whether certain states have to evolve first, to act as precursors for the next step in the evolutionary chain. We could visualize such scenarios like so:
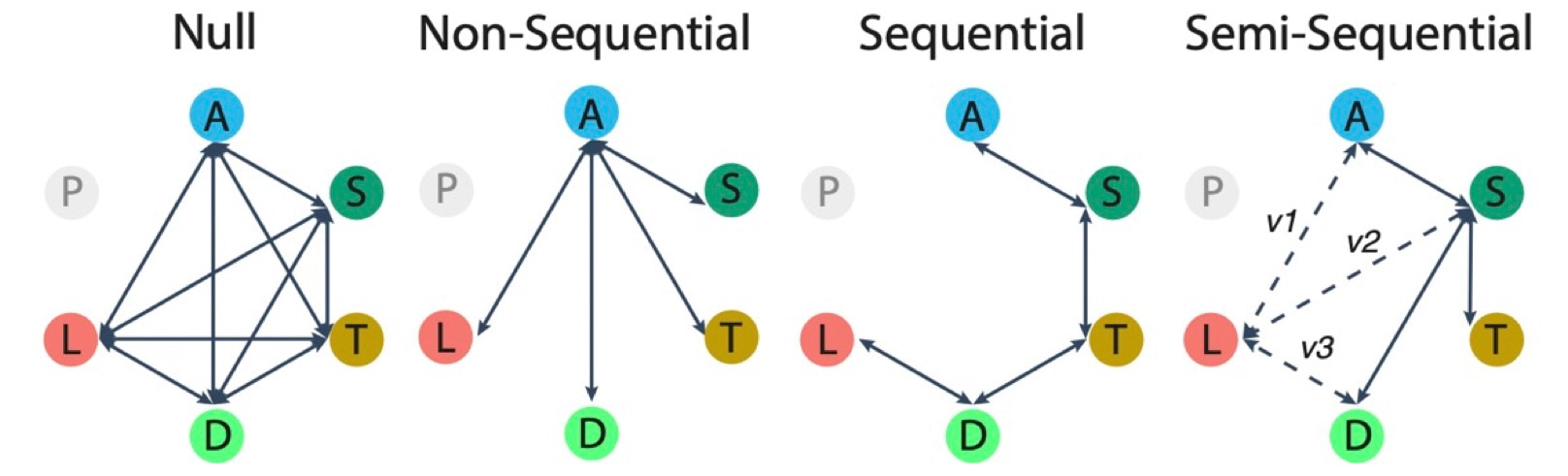
We found that in all three groups (frogs, salamanders and caecilians) there is structure to the transitions in states. That is, the “null” scenario was not among the best models. However, transitions are not strictly sequential either. A crucial sequential element is maintained in all three groups: the semi-terrestrial mode appears to have been a frequent precursor for direct-development.
We also found that despite more than 300 million years of evolution, each group contained substantial numbers of species (33–44%) that retained the most ancestral mode: a complex life cycle with aquatic larvae. The most frequent evolutionary transitions have been from the ancestral aquatic mode of reproduction to modes that are relatively uncommon in that group: live-bearing in caecilians, adults retaining larval features in salamanders, and frogs with terrestrial eggs but aquatic larvae.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in