Low-Temperature Electrolytes for Lithium-Ion Batteries: Current Challenges, Development, and Perspectives
Published in Chemistry, Materials, and Computational Sciences
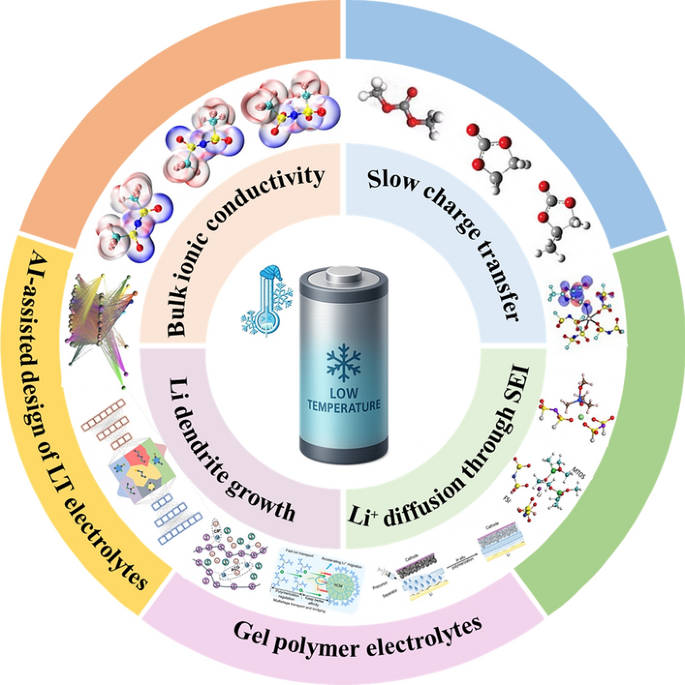
As electric vehicles, satellites and wearable electronics push into sub-zero environments, conventional lithium-ion batteries (LIBs) lose most of their energy and power, while lithium plating threatens safety. Now, researchers from Chang’an University and Queensland University of Technology, led by Professor Limin Geng, Professor Weijia Meng and Dr Jiaye Ye, have published a forward-looking review on low-temperature (LT) electrolytes that keep LIBs charging and discharging down to −80 °C. This work offers a systematic roadmap for next-generation energy-storage systems that thrive in the cold.
Why LT Electrolytes Matter
• Energy Efficiency: Rational molecular design slashes Li+ desolvation energy to <40 kJ mol-1, cutting polarization losses and eliminating external heaters that drain 10–20 % of pack energy.
• In-Battery Transport: High-entropy and weakly-solvating formulations maintain ionic conductivity >1 mS cm-1 at −60 °C, enabling 4 C charge rates without lithium plating.
• Extreme-Weather Applications: From Mars rovers to Arctic drones, LT electrolytes unlock reliable power where traditional LIBs cease to function.
Innovative Design and Features
• Electrolyte Families: The review covers ester-based (methyl acetate, ethyl difluoroacetate), ether-based (DOL/DME, THF, CPME), nitrile-based (fluoroacetonitrile) and gel-polymer systems, detailing how freezing point, dielectric constant and donor number dictate Li⁺ solvation structure.
• Functional Components: Dual-salt (LiFSI-LiDFOB), ternary-anion (LiPF6-LiTFSI-LiNO3) and AI-screened additives (LiTDI, NaPFO) are highlighted for building LiF- or Li3PO4-rich SEI/CEI layers that reduce interfacial resistance ten-fold.
• AI-Guided Formulation: Machine-learning models trained on >150 000 molecular candidates predict melting point, viscosity and LUMO energy within 5 K or 0.1 eV, accelerating electrolyte discovery from months to hours.
Applications and Future Outlook
• Multi-Level Screening: High-throughput DFT plus SHAP interpretability identifies dipole moment and molecular radius as key descriptors, delivering non-fluorinated ethers that cycle 300 times at −30 °C with 99 % capacity retention.
• Digital Logic Gates: LT gel-polymer electrolytes enable flexible printed-circuit modules that operate at −40 °C, providing a new route for cold-weather in-memory computing and IoT sensors.
• Artificial Interphases: From self-assembled NaPFO monolayers to organosilicon-rich SEIs, these nano-films suppress dendrites and raise Coulombic efficiency to 97.5 % at −60 °C.
• Challenges and Opportunities: The review pinpoints the need for standardized LT testing protocols, physics-informed neural networks that couple solvation structure to plating propensity, and automated robotic platforms that translate AI predictions into litre-scale synthesis. Future work will target high-entropy electrolytes, phase-diagram-guided formulations and cryogenic in-situ NMR to close the gap between lab demos and commercial 8 Ah pouch cells.
This comprehensive roadmap provides materials scientists, cell engineers and AI researchers with a common language for co-optimizing salts, solvents, additives and polymers for sub-zero operation. Stay tuned for more breakthroughs from Professor Limin Geng, Professor Weijia Meng and Dr Jiaye Ye!
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in