Magnetic aerogel for hazardous dye and toxic metals remediation
Published in Sustainability
Rapid urbanization and industry expansion have caused massive public health and water quality. As a result, various industries discharge their heavy metals and organic dyes into the aquatic systems without a proper purification process. Based on literature studies, hexavalent chromium [Cr(VI)] is intensively used in the tannery, textile and steel fabrication, while arsenic discharge into environmental surroundings via geothermal process and mineral deposits. The maximum permissible concentrations of arsenic and chromium regulated by the World Health Organization (WHO) are 10 and 50 ppb in drinking water. An additionally, dyes originate from various industrial activities such as textile, cosmetics, leather, food, pharmaceutical, paper productions (pulp), paint and varnish. These discharged dyeing effluents are carcinogenic, mutagenic which are highly toxic to humans and ecological organisms. Hence, it’s vital to treat them before their discharge. In this regard, herein we fabricated a promising adsorbent of magnetic aerogel (PEI-h-BNNSs@Fe3O4NPs) for environmental remediation with high adsorption efficiency.
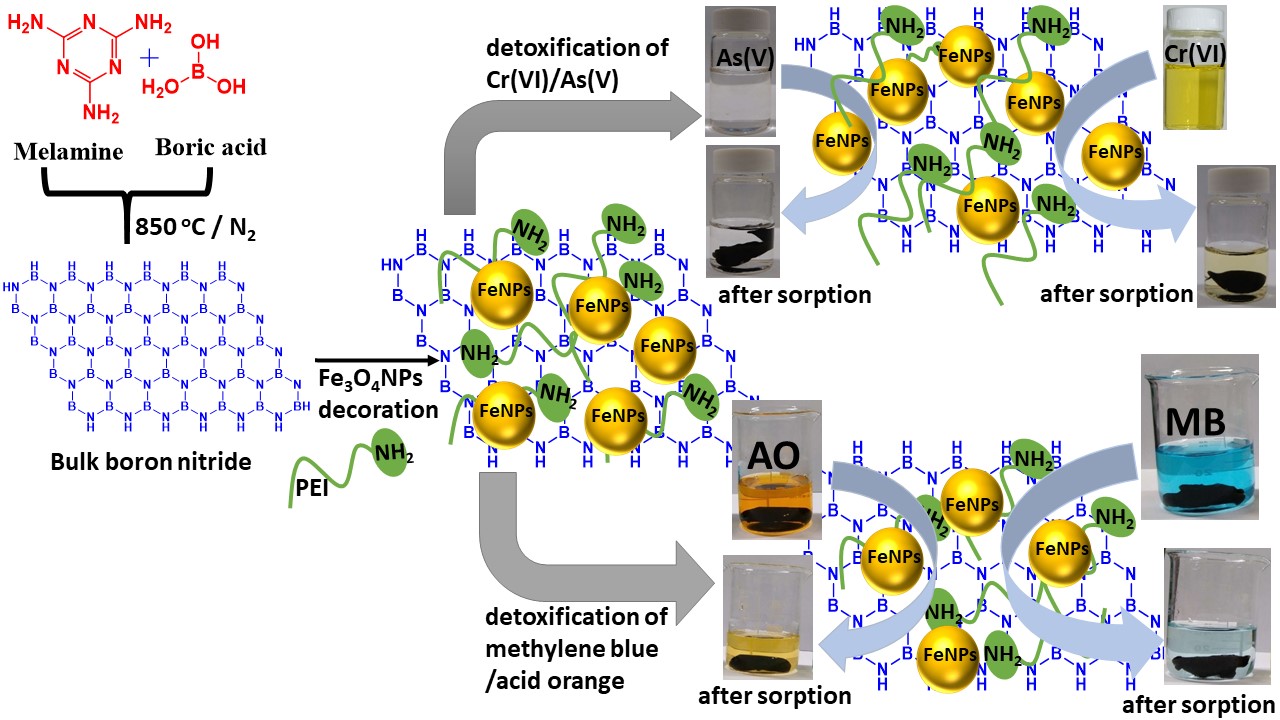
Figure 1. Schematic illustration of Fe3O4 NPs-decorated PVA-modified PEI-h-BNNSs aerogel (MHAs, adsorbent) for efficiently capturing of heavy metals and organic dyes from an aqueous solution.
The thermal poly condensation of melamine and boric acid, pyrolysis of the resultant products which allowed to exfoliate by ultra-sonication process further functionalization with PEI-mediated modification of h-BNNSs. The as formed PEI-h-BNNSs allowed in-situ formation of magnetite nanoparticles (Fe3O4 NPs) decorated on their surfaces, which are turned to be PEI-h-BNNSs@Fe3O4 NPs. The lyophilization treatment of PEI-h-BNNSs@Fe3O4 NPs-loaded PVA hydrogels generated the magnetic hybrid aerogel (MHAs) with large surface area to volume ratio and good super-paramagnetic behavior. The as-made MHAs exhibited a three-dimensional porous structure with diverse functional groups (−N, −NH, −NH2, and −OH) and zero net charge.
An additionally, incorporating magnetic nanoparticle into aerogels (3-dimensional porous structure) had the added advantages of simplicity, low cost and rapidity in the recycling process. These features enabled the as-made MHAs serve as a powerful adsorbent to remove Cr(VI), As(V), MB and AO from an aqueous solution and their adsorption behavior which follows Freundlich isotherm model and a pseudo-second-order model. In contrast to the PEI-h-BNNSs-loaded PVA aerogels, Fe3O4 NPs-loaded PVA aerogels and other previously reported adsorbents, the MHAs provide numerous distinct advantages, including (1) the presence of highly mesoporous structures with a large specific surface area of 104.6 m2 g-1, (2) the possession of diverse and abundant functional groups (−N, −NH, −NH2, and −OH) on the surface, (3) outstanding adsorption capacity for capturing of Cr(VI) (833 mg g-1), As(V) (426 mg g-1), MB (415 mg g-1) and AO (286 mg g-1), (4) in-situ reduction of Cr(VI) to Cr(III) and As(V) to As(III), (5) The adsorption performance of MHAs allows the removal of >95% for Cr(VI) and As(V) in contaminated soil-sludge samples, (6) more than three successive adsorption-desorption cycles for >80% uptake of Cr(VI), As(V), MB, and AO and (7) the accessible collection of MHAs by applying an external magnetic field. Accordingly, our research work discloses that, the as-made aerogels composed of magnetic nanoparticle and h-BNNSs-based materials cross-linked with PVA polymers have great potential candidate (adsorbent) in large-scale of water purification process.
To read more about our work at Nature Portfolio Journal clean water: https://www.nature.com/articles/s41545-022-00175-0
Follow the Topic
-
npj Clean Water

This journal publishes high-quality papers which report cutting-edge science, technology, application, policy and societal issues that contribute towards a more sustainable supply of clean water.
What are SDG Topics?
An introduction to Sustainable Development Goals (SDGs) Topics and their role in highlighting sustainable development research.
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Advancing Artificial Intelligence Innovations for Resilient and Sustainable Clean Water: Novel Methodologies and Case Studies
Publishing Model: Open Access
Deadline: Feb 28, 2026
Advances in Smart Water Systems: From Source to Tap and Beyond
Publishing Model: Open Access
Deadline: Feb 28, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in