Maintaining normal levels of tryptophan is beneficial for the gut microbiota of patients with alcohol-associated liver disease.
Published in Healthcare & Nursing, Bioengineering & Biotechnology, and Microbiology

Alcohol-associated liver disease (ALD) is a broad term encompassing a spectrum of liver pathologies that result from excessive and chronic alcohol intake, named alcohol use disorder (AUD). In ALD patients, liver injury progresses from steatosis, inflammation (hepatitis), fibrosis, cirrhosis, and its complications, including liver cancer1. The therapeutic options in both AUD and ALD, notably to avoid liver disease progression, are limited and mainly depend on alcohol withdrawal, which is often unsuccessful in the majority of patients2,3. The intestinal microbiota (IM) plays a role in the severity of alcohol-associated liver disease. Modifying severe alcohol-associated hepatitis (AH) dysbiosis improves liver injury through tryptophan (Trp) metabolites and the aryl hydrocarbon receptor (AhR). However, the impact of Trp as a precursor of indole ligands on ALD IM has not been thoroughly investigated.
How to explore the effects of Trp on the gut microbiota of ALD patients?
We used an in vitro microbiota modelling system, named minibioreactor arrays (MBRAs), which allows stable cultivation of human IM and to investigate the direct modulatory role played by Trp and alcohol4,5 .Fecal samples from AUD patients with sAH (n=2) or with noAH (n=2) were transferred to MBRAs chambers with three Trp concentrations (low: 8mg/L, normal: 24mg/L and high: 72mg/L) for 4 days. Subsequently, alcohol was introduced into the system for 5 days (50 mM ethanol/Day). Finally, alcohol was removed and the cultures were maintained for an additional 5 days. Fecal samples and supernatants were collected to explore the IM changes and the AhR activation.
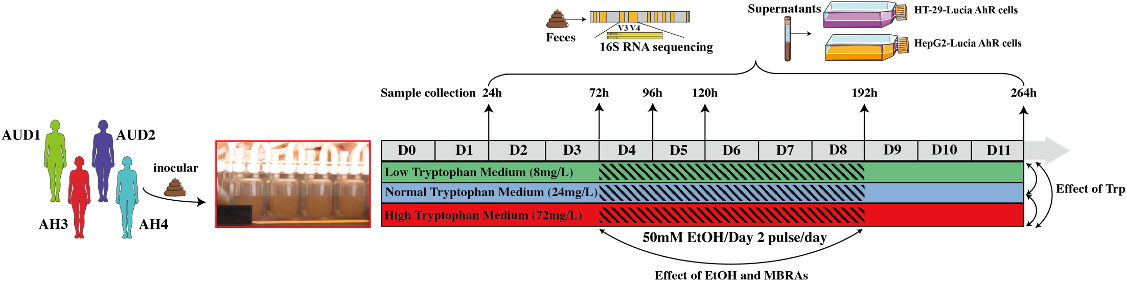
Low and high Trp levels increase pathogens
During cultivation, MBRA is stable to keep each individual fecal community, which permits exploring the effect of Trp. The results show that changes in Trp concentration do not alter microbial diversity or composition, as indicated by alpha and beta diversity. However, upon comparing the differential bacteria between the different Trp concentrations in AUD samples, it was observed that low Trp concentration treatment resulted in differences with 14 genera, and 2 phyla compared to normal Trp concentration. In contrast, only 4 genera differed between the normal and high Trp concentration conditions. In AUD and AH, both high and low concentrations of Trp increased the levels of Escherichia Shigella, which is considered as intestinal pathogen associated with various diseases and related to pro-inflammatory and metabolic disorders6,7.
Alcohol induces greater changes in AUD IM and increases Escherichia-Shigella levels.
AUD patients do not have liver damage compared to AH patients although both are heavy drinkers. However, alcohol induced more changes in the relative abundance of members of the AUD microbiota community (7 genera, 2 phyla) compared to those in AH (4 genera). What’s more, In AUD IM with normal Trp concentration,alcohol increased the levels of pathobionts, including Escherichia Shigella and Paeniclostridium, while decreasing the levels of bacteria such as Bacteroides. Therefore, alcohol still affects the IM of AUD patients, increasing the expression of some pathobionts, but its impact on AH IM appears minimal in normal Trp conditions.
In addition, with alcohol exposure, only a few bacteria are affected by Trp concentration in AUD and AH IM. When alcohol and Trp are added simultaneously, the influence of different Trp concentrations on IM is minimal.
The IM did not recover after alcohol withdrawal
To further assess the impact of alcohol on the AUD and AH microbiota, we removed alcohol from the culture medium and conducted a 4-day recovery period culture. For the overall IM composition, there were no differences between the IM during the recovery period after alcohol removal and the initial state. However, Escherichia-Shigella, Paenibacillus_lautus, Tissierella, Firmicutes, and Desulfobacterota remained at high levels in AUD IM under normal Trp concentration. Conversely, in the AH IM, a few differential bacteria were found after alcohol withdrawal as compared to the initial states. Overall, AUD IM did not return to its pre-alcohol state after alcohol withdrawal under normal Trp concentration.
Normal and high concentrations of Trp supplementation activate AhR
We collected fecal supernatant from MBRAs to treat HT-29 AhR-Lucia and HepG2 AhR-Lucia cells to test the activation of AhR. We found that metabolites of AH IM decreased AhR activity in HT-29 cells and hepatocytes but can be reversed by normal concentrations of Trp in the presence of alcohol or not. Conversely, activation of AhR by AUD IM seems to depend on high Trp level with alcohol or normal Trp level without alcohol.
In conclusion, our study modeled the evolution of IM from patients with AUD and AH in vitro and showed that AUD IM’s is more dynamic in terms of changes as compared to AH. Therefore, with the advantage of MBRA in stabilizing IM and the exclusion of other interfering factors, our study suggests that Trp supplementation to restore the recommended nutritional intake in AUD and AH patients may be beneficial and set the basis for further studies in clinical settings.
1 Gao, B. & Bataller, R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141, 1572-1585, 2011.
2 Mackowiak, B., Fu, Y., Maccioni, L. & Gao, B. Alcohol-associated liver disease. J Clin Invest 134 , 2024.
3 Mirijello, A. et al. Identification and management of alcohol withdrawal syndrome. Drugs 75, 353-365, 2015.
4 Auchtung, J. M., Robinson, C. D., Farrell, K. & Britton, R. A. MiniBioReactor Arrays (MBRAs) as a Tool for Studying C. difficile Physiology in the Presence of a Complex Community. Methods in molecular biology (Clifton, N.J.) 1476, 235-258, 2016.
5 Auchtung, J. M., Robinson, C. D. & Britton, R. A. Cultivation of stable, reproducible microbial communities from different fecal donors using minibioreactor arrays (MBRAs). Microbiome 3, 42, 2015.
6 Zhao, J. et al. Expansion of Escherichia-Shigella in Gut Is Associated with the Onset and Response to Immunosuppressive Therapy of IgA Nephropathy. J Am Soc Nephrol 33, 2276-2292, 2022.
7 Kong, C., Gao, R., Yan, X., Huang, L. & Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 60, 175-184, 2019.
Follow the Topic
-
npj Biofilms and Microbiomes

The aim of this journal is to serve as a comprehensive platform to promote biofilms and microbiomes research across a wide spectrum of scientific disciplines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Natural bioactives, Gut microbiome, and human metabolism
Publishing Model: Open Access
Deadline: Feb 20, 2026
Harnessing plant microbiomes to improve performance and mechanistic understanding
Publishing Model: Open Access
Deadline: Jun 01, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in