Membrane topography variations cause apparent protein clustering
Published in Physics, Protocols & Methods, and Cell & Molecular Biology
Most membrane associated molecules are reported to form clusters which biologically seems very unlikely, so we decided to investigate this issue. We found that apparent clustering of membrane proteins occurs upon failing to account for membrane topography variations. This we demonstrate and present a remedy for. Our data suggest that the high incidence of clustering reported misrepresents biology and clouds our conceptional understanding of cellular processes.
Detailed knowledge on the organisation of plasma membrane lipids and proteins is crucial to understand cellular processes like adhesion, endocytosis, migration, secretion and the initiation of cell signalling. This knowledge also helps us understand disease mechanisms such as viral infection and egression of host-synthesised new viral particles, the interaction of amyloids, found in e.g. Alzheimer’s and diabetes, with membranes and aberrant signalling responses as seen in cancer. An important aspect to address is whether the membrane components are clustered, which covers dimers to macroscopic assemblies, and how the degree of clustering varies with changing cellular activities.
A tool commonly used to study the organisation of membrane molecules is single-molecule localisation microscopy (SMLM) where the positions of individual molecules can be determined at high accuracy by allowing only a fraction of the fluorophores to emit photons at any given time. This achieves resolution in the 10-20 nm range and SMLM belongs to the family of super resolution microscopy techniques whose development was awarded with the Nobel Prize in 2014.
We introduce and consider an often overlooked biological reason for the reported high degree of clustering of membrane proteins – the presence of variations in membrane topography. Membrane topography features include protrusions like filopodia and lamellipodia as well as invaginations like caveolae and clathrin coated pits. None of these are static features and in combination with general membrane folding and undulations they are responsible for membrane topography variations. In SMLM, TIRF microscopy is commonly used which means that only fluorophores that are close to the surface to which the cells adhere will be subjected to excitation and hence give rise to a fluorescence signal. I. e. what is perceived as a cluster may well be parts of the cell that are closer to the coverslip.
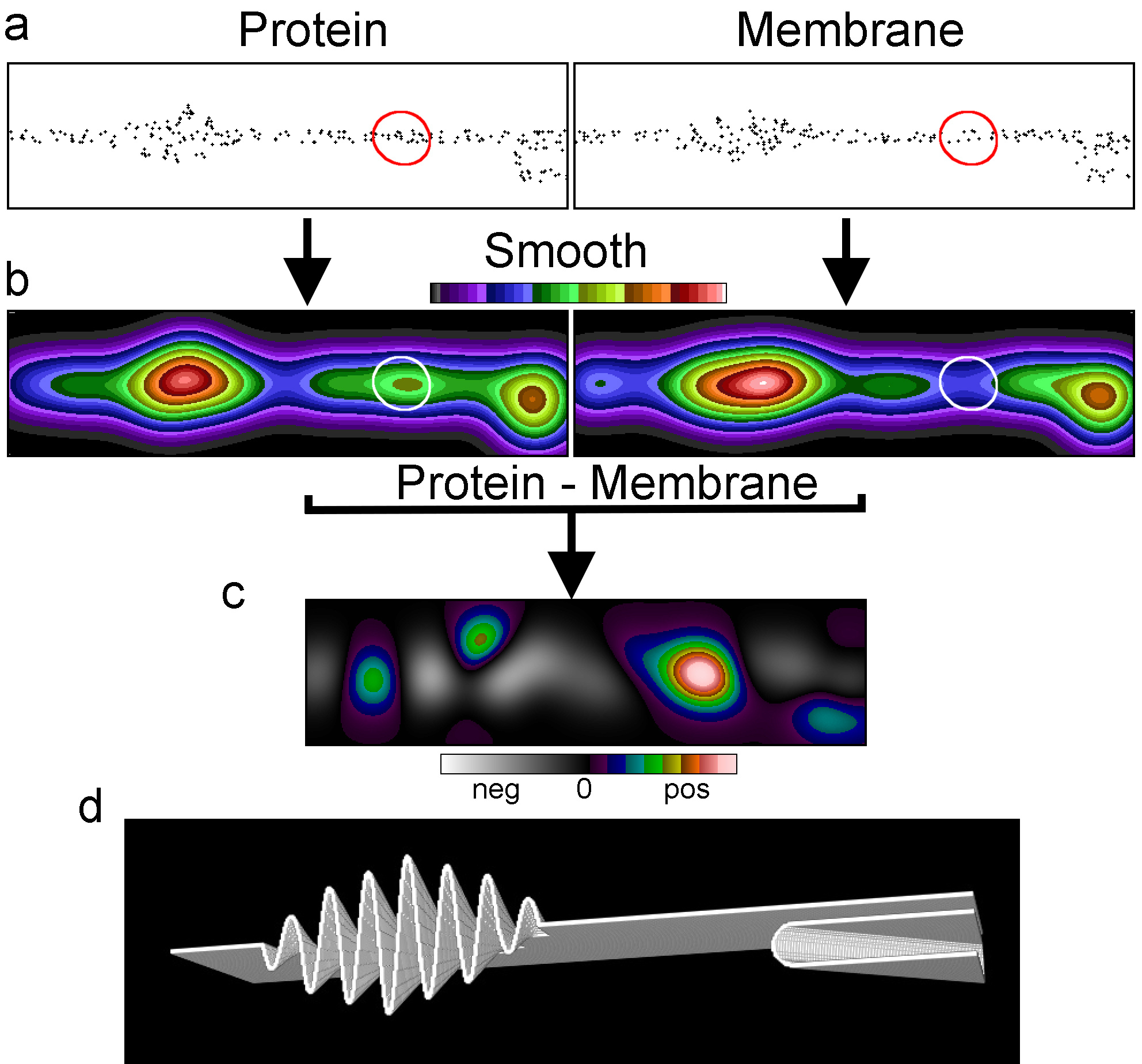
Fig. 1 Membrane topography variations can cause the appearance of clustering. (a) Membrane cross section of a simulated SMLM dataset of a membrane protein and a randomly distributed membrane marker. (b) The datasets after smoothing with a Gaussian filter and the images normalised to the same mean intensity, displayed with the same intensity range. (c) The difference image between the two normalized Gaussian filtered images with the display stretched and zero at the centre. (d) The rendered membrane with folds used for the simulations.
Using SMLM simulations of a randomly distributed membrane marker in a convoluted membrane, we demonstrate that the marker being perceived as clustered could be a consequence of variations in membrane topography (Fig. 1). As the topography varies, the area of membrane represented by each pixel also varies and the local density of randomly distributed molecules will follow this variation. This is clearer when the localisations are smoothed into an image. In this example there appears to be two clusters where the membrane density per pixel is high. When simulating a protein with one small cluster (marked with a circle) and an otherwise random distribution in the same convoluted membrane as the membrane marker there appears to be three clusters. However, two of these coincide with the membrane-rich areas. This suggested to us that genuine and apparent clusters could be differentiated by image intensity distribution analysis after subtraction. After normalising the intensities of the two images, the membrane marker image was subtracted from the protein image to generate a difference image. The result of the subtraction was that the genuine cluster became prominent, whereas the other two clusters disappeared.
Next, we performed dual colour live cell SMLM on the distributions of the transferrin receptor (TfR) or the GPI-anchored protein CD59 in HT29 cells using the fluorophore DiI as a membrane marker. Interestingly, the two cluster analysis methods nearest neighbour analysis and pair correlation suggested that all three molecules were clustered. Converting the localisations into images supported this conclusion (Fig. 2). However, when performing intensity distribution analyses using the DiI image to factor out topography variations, CD59 clusters were no longer visible, suggesting that the clustering reported by the other methods for CD59 is an artefact. The TfR clusters, on the other hand, persisted after the topography variations were factored out. This demonstrates that without knowing the membrane topography variations, it is not advisable to claim that a membrane molecule is clustered.
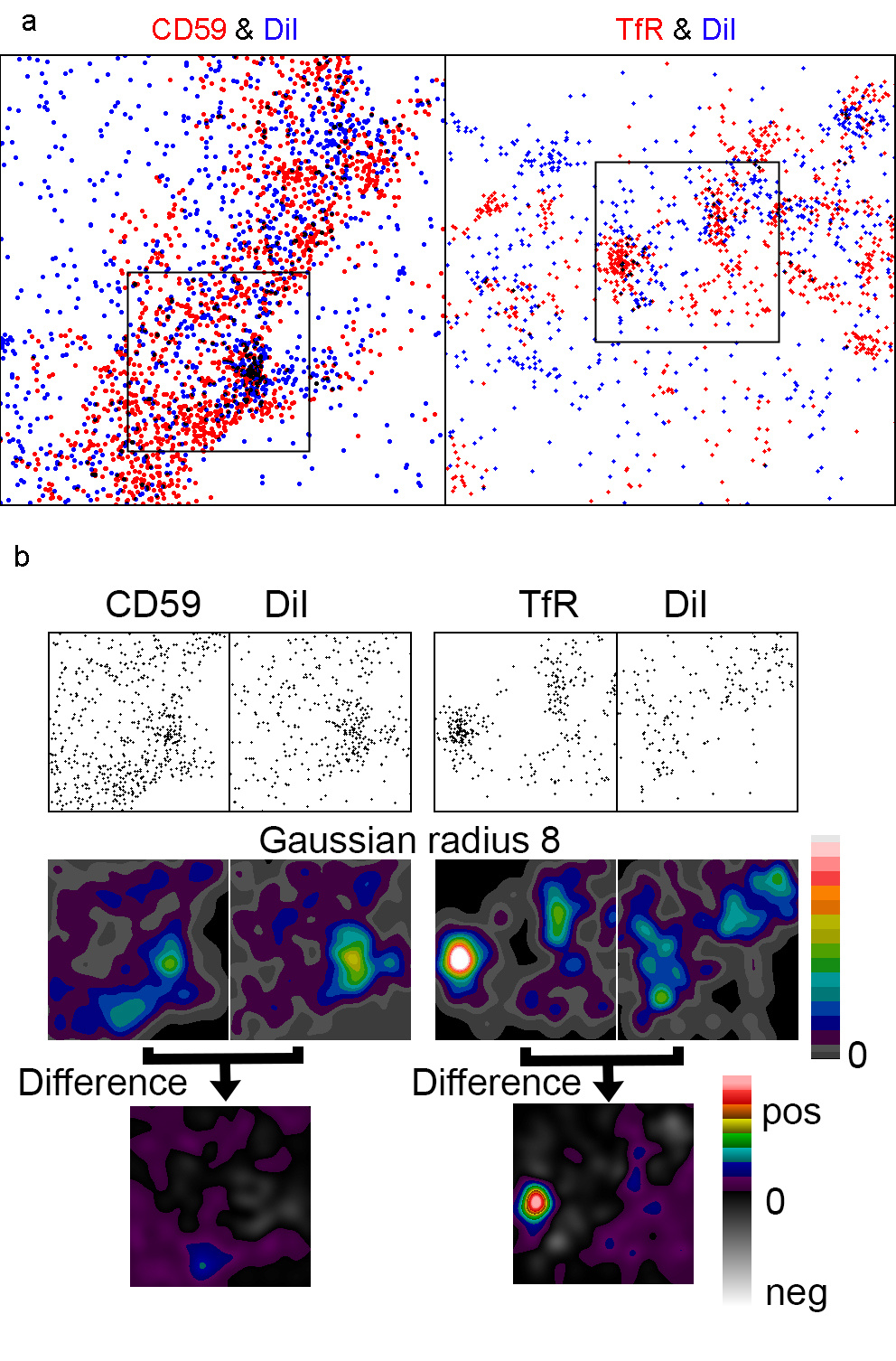
Fig. 2 Live cell dual colour SMLM in HT29 cells. (a) 2.5x2.5 mm SMLM 7s snapshots of DiI and CD59 or the TfR with the proteins identified with a-Alexa Fluor-647. (b) 1x1 mm regions from the larger images. Original and Gaussian filtered versions of all four images were normalised to the same mean intensity and displayed with the same intensity range. The final pair of images shows the result of subtraction of the DiI image from that of the relevant protein.
In summary, we demonstrate that the basal side of adhered cells have substantial variations in membrane topography which can create the appearance of clustering and we introduce a method for differentiating between these artefactual clusters and genuine clusters. The problem and the solution apply more widely than SMLM - whenever a subresolution structure variably occupies voxels. We recommend that variations in membrane topography are excluded before a molecule is reported to be clustered since the existence, the extent of and changes in clustering are important for the interpretation of biology.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in