Mesenchymal stem cells exert renoprotection via extracellular vesicle-mediated modulation of M2 macrophages and spleen-kidney network
Published in Healthcare & Nursing

Key Points
- Adipose mesenchymal stem cells (ASCs) dramatically improved severe nephritis.
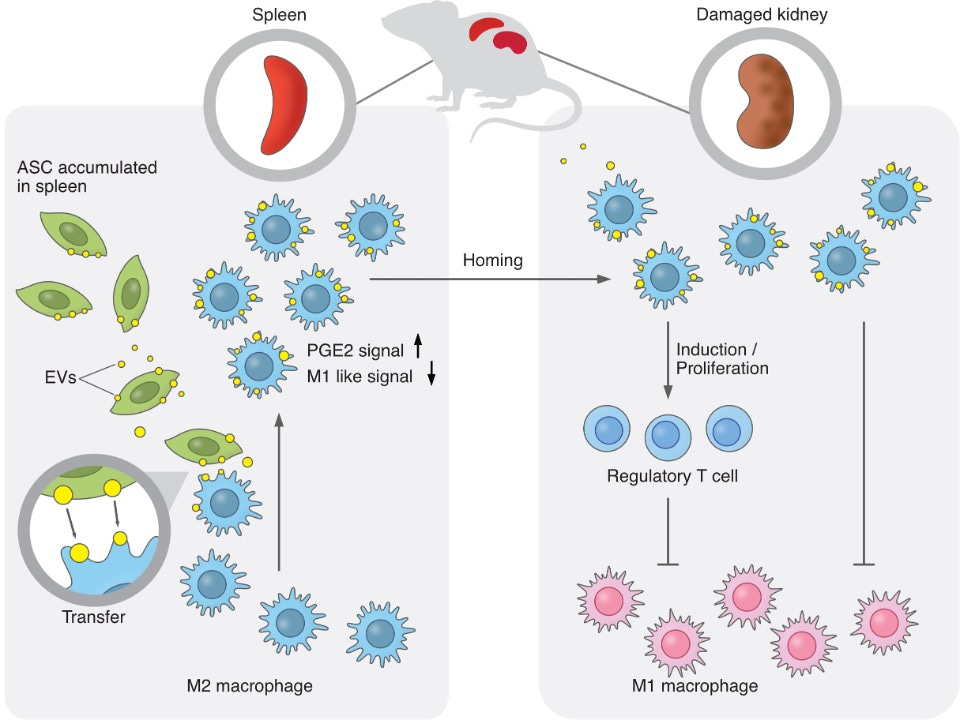
- ASC-derived extracellular vesicles (EVs) were transferred to M2 macrophages, which entered the bloodstream from the spleen. EVs induced the transcriptomic signatures of hyperpolarization and PGE2 stimulation in M2 macrophages and ameliorated glomerulonephritis.
- This study presents a possibility for a treatment that would not involve the direct administration of adipose-derived mesenchymal stem cells into the body.
Summary
Adipose-derived mesenchymal stem cells (ASCs) have shown therapeutic potentials against refractory diseases. However, the detailed therapeutic mechanisms remain unclear. Here, we report the therapeutic actions of human ASCs in nephritis, focusing on cellular dynamics and multi-organ networks. Intravenously-administered ASCs accumulated in the spleen but not kidneys. Nevertheless, ASCs increased M2 macrophages and Tregs in kidneys and drove strong renoprotection. Splenectomy abolished these therapeutic effects. ASC-derived extracellular vesicles (EVs) were transferred to M2 macrophages, which entered the bloodstream from the spleen. EVs induced the transcriptomic signatures of hyperpolarization and PGE2 stimulation in M2 macrophages and ameliorated glomerulonephritis. ASCs, ASC-derived EVs, and EV-transferred M2 macrophages enhanced Treg induction. These findings suggest that EV transfer from spleen-accumulated ASCs to M2 macrophages and subsequent modulation of the renal immune-environment underlie the renoprotective effects of ASCs. Our results provide insights into the therapeutic actions of ASCs, focusing on EV-mediated modulation of macrophages and the spleen-kidney immune network.
Research Background
Mesenchymal stem cells (MSCs) are known to have outstanding regenerative and immunomodulatory properties, and MSCs are expected to be therapeutic for refractory diseases that cannot be treated with existing drugs. Therefore, more than 1,000 clinical trials have been conducted globally to demonstrate the efficacy of MSCs in the treatment of refractory diseases. We are also conducting clinical studies using adipose-derived MSCs (ASCs) for refractory IgA nephropathy. Although MSCs have attracted attention as a new regenerative medicine due to their high therapeutic efficacy and their therapeutic mechanism has been studied intensively, no single molecule has been able to fully explain their mechanism of action. Elucidating the mechanism of action will not only further enhance the therapeutic effects of MSCs, but may also provide clues to drug discovery that could enable treatment without the administration of cells. Up to now, most MSCs studies in animal models have used mouse or rat MSCs, but it is assumed that the actions and mechanisms of MSCs will change with different animal species. Therefore, in this study, we aimed to perform an analysis by using human MSCs to directly address the clinical significance of ASCs. We took a different approach from previous mechanistic analyses based on the molecules, and clarified the action of ASCs by focusing on the dynamics of ASCs in the body and their cell-to-cell communication.
Research Results
We report the therapeutic potential of human ASCs for nephritis, focusing on in vivo cellular dynamics and multi-organ networks. Intravenously-administered ASCs accumulated in the spleen but not the kidneys. Nevertheless, ASCs increased M2 macrophages and Tregs in the damaged kidney, decreased neutrophils and M1 macrophages, and drove strong renoprotection. Splenectomy abolished these therapeutic effects. Flow cytometry analysis revealed that ASC-derived extracellular vesicles (EVs) were specifically transferred to M2 macrophages, and intravital microscopy imaging demonstrated that EV-transferred macrophages entered the bloodstream from the spleen. EVs induced the transcriptomic signatures of macrophage activation and PGE2 stimulation in M2 macrophages and partially ameliorated glomerulonephritis. Furthermore, ASCs, ASC-derived EVs, and EV-transferred M2 macrophages enhanced Treg induction in T cells. These findings collectively suggest that specific EV transfer from spleen-accumulated ASCs to M2 macrophages and subsequent modulation of the kidney immune environment underlie the renoprotective effects of ASCs. Our results provide insights into the therapeutic mechanisms of ASCs, focusing on EV-mediated modulation of macrophages and the spleen-kidney immune network, which may lead to the maximized potential of cell therapies in clinical settings.
Research Summary and Future Perspective
Our laboratory has focused on adipose-derived mesenchymal stem cells (ASCs) because they are easier to harvest than bone marrow and have superior proliferative potential. In this study, we found that ASCs have a higher immunomodulatory and organ-protective potential than bone marrow-derived MSCs. In the future, we hope to apply ASCs clinically as a novel treatment for various refractory inflammatory diseases in addition to nephritis. We will apply the action of ASCs elucidated in this study to therapies that enhance the therapeutic potential of ASCs, and further advance them to new therapies using EVs that would not involve the direct administration of adipose-derived mesenchymal stem cells into the body.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in