Methylmalonic acid is exhausting! The story of an age-related metabolite that suppresses anti-tumor immunity
Published in Cancer

What is methylmalonic acid and what led us to pursue this study?
As a simple definition, methylmalonic acid (MMA) is a toxic metabolite. It is a byproduct of propionate metabolism, which is a pathway that takes place in the mitochondria. This pathway involves the catabolism of branched chain amino acids and odd chain fatty acids to eventually form succinyl-CoA, an intermediate in the tricarboxylic acid cycle. MMA levels are generally low under normal physiological conditions, but increased levels can come from different sources, such as vitamin B12 deficiency, genetic mutations in enzymes related to the propionate pathway, the gut microbiota, and aggressive cancers1.
Gomes et al2 demonstrated that MMA levels are also increased with age and that high MMA levels promote tumor progression by reprogramming lung and breast cancer cells to adopt aggressive properties, such as increased metastasis. MMA not only had these extrinsic effects but had intrinsic effects within cancer cells themselves. Gomes et al3 showed that, compared to nonmetastatic breast cancer cells, metastatic breast cancer cells downregulate an enzyme of the propionate metabolism pathway, methylmalonyl-CoA epimerase, which leads to an intracellular accumulation of MMA and fuels metastasis. Thus, the direct effect of MMA on tumor cells to promote tumor progression is two-fold.
However, a question remained - does MMA also promote tumor progression by having suppressive effects on anti-tumor immunity? The proliferation and function of CD8+ T cells, adaptive immune cells important for anti-tumor immunity, is known to decline with age4 and in conditions of vitamin B12 deficiency5 which are the two most well-established conditions that lead to increased MMA levels. Thus, we pursued this study to investigate whether a cause-and-effect relationship exists between increased MMA levels and CD8+ T cell dysfunction.
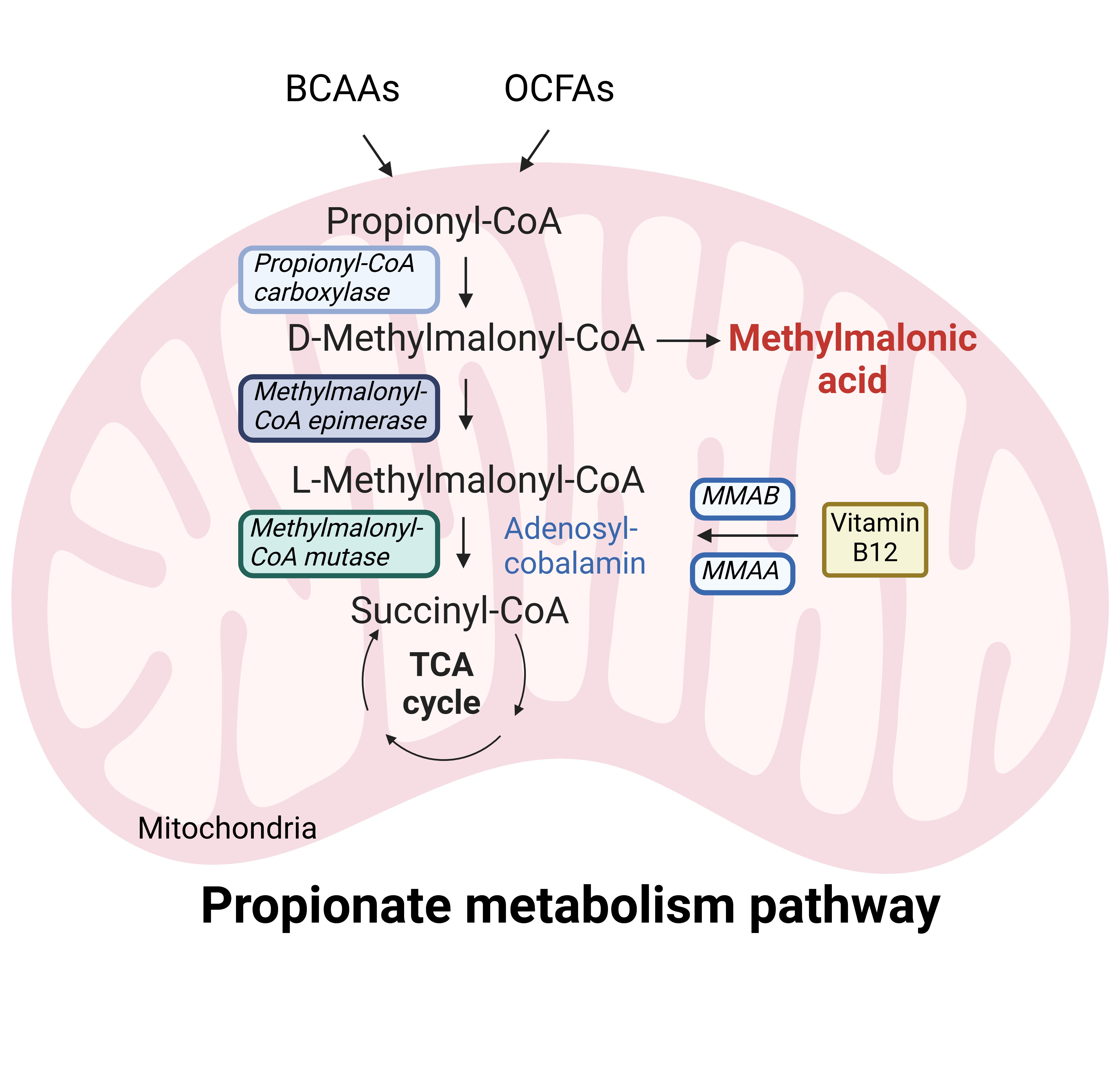
Abbreviations: BCAAs, branched chain amino acids; OCFAs, odd chain fatty acids; MMAA, metabolism of cobalamin associated A; MMAB, metabolism of cobalamin associated B; TCA cycle, tricarboxylic acid cycle.
Our experimental approach
To investigate this, we isolated primary CD8+ T cells from wild-type C57BL6 mice and activated them in the presence of a high, physiologically relevant concentration of MMA2 for three days in vitro. Validation in vivo was performed using the administration of MMA by intraperitoneal injections twice daily for four weeks in either non-tumor bearing mice or mice challenged with luciferase-expressing lung cancer cells.
A summary of our findings
We found that treatment of primary CD8+ T cells with MMA promoted an exhausted phenotype characterized by decreased proliferation, activation, and effector functions. It increased the expression of the exhaustion markers, PD-1, CD38, and TOX, but not TIM-3 or LAG-3. MMA caused metabolic defects, especially in the TCA cycle, and decreased mitochondrial function while increasing reactive oxygen species production. This drove the suppression of anti-tumor immunity in vitro and in vivo. Thus, MMA is a potent regulator of CD8+ T cell fate and function.
Take-home message and future perspectives
In summary, our findings show that high levels of MMA induced dysfunction and exhaustion in CD8+ T cells, thereby halting anti-tumor immunity. With that, we can add MMA to the growing list of metabolites that have immunomodulatory functions.
So, what is the significance of these findings? We anticipate that these studies have the potential to change clinical practice by recognizing high levels of MMA as a biomarker or modifiable risk factor to prevent immune suppression or tumor progression.
Yet, important questions remain - what is the mechanism of action by which MMA exerts its effects in CD8+ T cells? What are the effects of MMA on other immune cell populations, like CD4+ T cells, B cells, or NK cells? Considering that MMA levels increase under conditions of vitamin B12 deficiency, would vitamin B12 supplementation rescue the effects seen with high MMA? Defining these follow-up questions will provide deeper insights into the full spectrum of the role of MMA in whole body physiology (e.g., vitamin B12 deficiency, immune deregulation) and tumorigenesis.

For more information, please check out our full article linked here or the Gomes Lab website.
References
- Tejero J, Lazure F, Gomes AP. Methylmalonic acid in aging and disease. Trends Endocrinol Metab. 2024;35(3):188-200. doi:10.1016/j.tem.2023.11.001
- Gomes AP, Ilter D, Low V, et al. Age-induced accumulation of methylmalonic acid promotes tumour progression. Nature. 2020;585(7824):283-287. doi:10.1038/s41586-020-2630-0
- Gomes AP, Ilter D, Low V, et al. Altered propionate metabolism contributes to tumour progression and aggressiveness. Nat Metab. 2022;4(4):435-443. doi:10.1038/s42255-022-00553-5
- Han S, Georgiev P, Ringel AE, Sharpe AH, Haigis MC. Age-associated remodeling of T cell immunity and metabolism. Cell Metab. 2023;35(1):36-55. doi:10.1016/j.cmet.2022.11.005
- Tamura J, Kubota K, Murakami H, et al. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol. 1999;116(1):28-32. doi:10.1046/j.1365-2249.1999.00870.x
Follow the Topic
-
Oncogene

This journal aims to make substantial advances in our knowledge of processes that contribute to cancer by publishing outstanding research.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in