Minimally invasive testing using molecular approaches for endometrial cancer diagnosis: the game-changer in cost-effective detection among women with postmenopausal bleeding
Published in Healthcare & Nursing

Early detection of endometrial cancer: overcoming current limitations with emerging approaches
Endometrial cancer is increasing mainly due to metabolic syndrome, obesity, and ageing population. While 90% of women with endometrial cancer experience abnormal uterine bleeding, only a mere 9% of those with postmenopausal bleeding are diagnosed with this cancer. Early detection is crucial for the effective treatment of endometrial cancer, and thus, all women with postmenopausal bleeding require further evaluation to early identify and treat this condition.
Transvaginal ultrasound and endometrial sampling have long been the go-to methods for diagnosing endometrial cancer. Still, they present some limitations: ultrasound suffers from poor specificity while the pipelle biopsy often fails, suffers from misclassification on grade and histology, and blind sampling can lead to false negatives. Cutting-edge molecular approaches utilizing genomics, epigenomics, and proteomics are on the horizon, promising to revolutionize endometrial cancer detection and help us catch this stealthy disease early.
These new approaches benefit from the anatomical continuity of the uterine cavity with the cervix and provide high sensitivity and specificity, thus offering a promising new horizon. However, the implementation of novel diagnostic approaches is associated with increased costs, so it is essential to compare the relative costs and health effects of both strategies to determine which is more efficient before implementation in healthcare plans.
Methodological approach
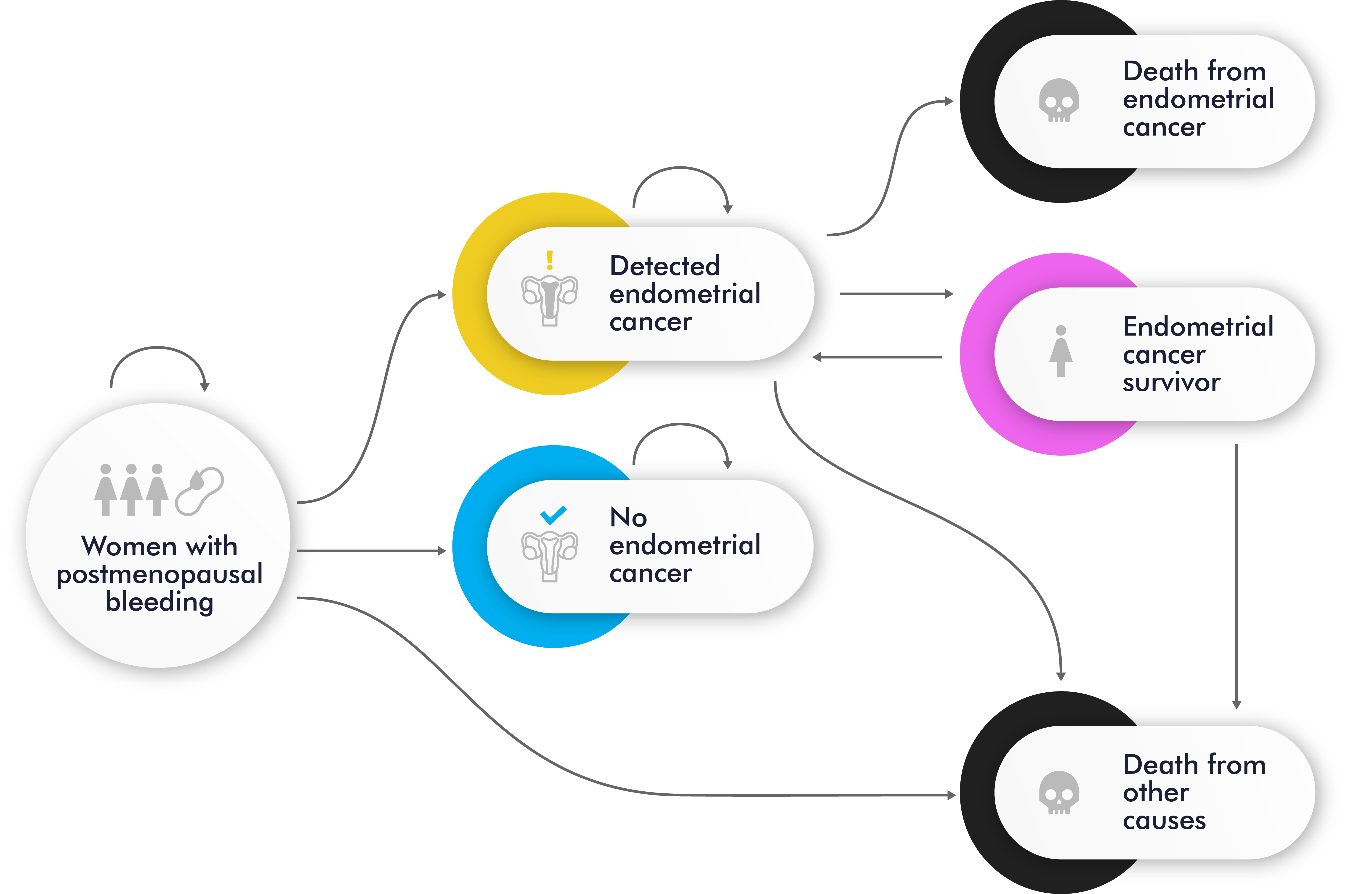
To address this issue, we performed a model-based cost-effectiveness analysis of two early detection strategies for endometrial cancer detection in women with postmenopausal bleeding: the current standard of care and a novel molecular strategy that uses a cervical cytology sample and/or endometrial sample for molecular testing. Figure 1 shows the Markov cohort model with 6 mutually exclusive health states with diagnostic strategies diagrams for endometrial cancer: postmenopausal bleeding (initial state for all women), detected endometrial cancer, no detected endometrial cancer, endometrial cancer survivor, death from endometrial cancer, and death from other causes.
Figure 1. Markov Model: state transition diagram.
The model predicts the number of hysterectomies, lifetime expectancy, quality-adjusted life years, endometrial cancer prevalence, incidence, and mortality, as well as the lifetime costs of screening, diagnosis, and treatment. The incremental cost-effectiveness ratio is used to measure cost-effectiveness. Several sensitivity analyses were conducted to identify the key parameters that could affect the results and to determine the robustness of the findings.
Key findings and take-home message
The diagnostic strategy to assess postmenopausal bleeding using molecular testing is cost-saving compared to the usual diagnostic strategy at a given cost of 310€ per molecular test.
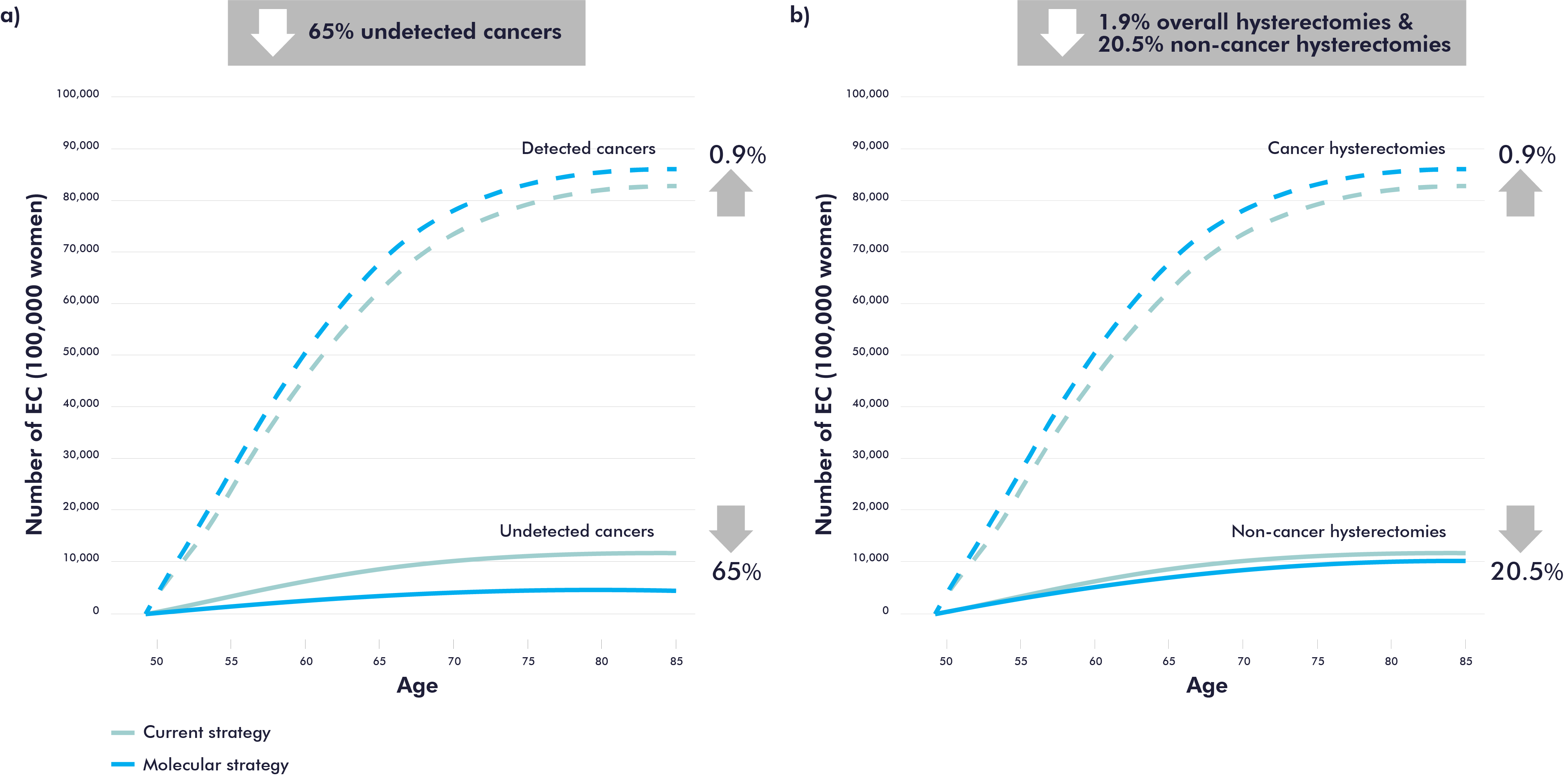
Furthermore, the results suggest that the molecular strategy is also more effective as molecular testing can potentially decrease the number of undetected endometrial cancer by 65% (Figure 2a), which can lead to early detection and better patient outcomes. Molecular testing has the potential to reduce the number of hysterectomies by an average of 20.5% in women with no endometrial cancer, with greater reductions at older ages (Figure 2b).
Figure 2. Health benefits results: reduction of undetected EC and in the number of hysterectomies.
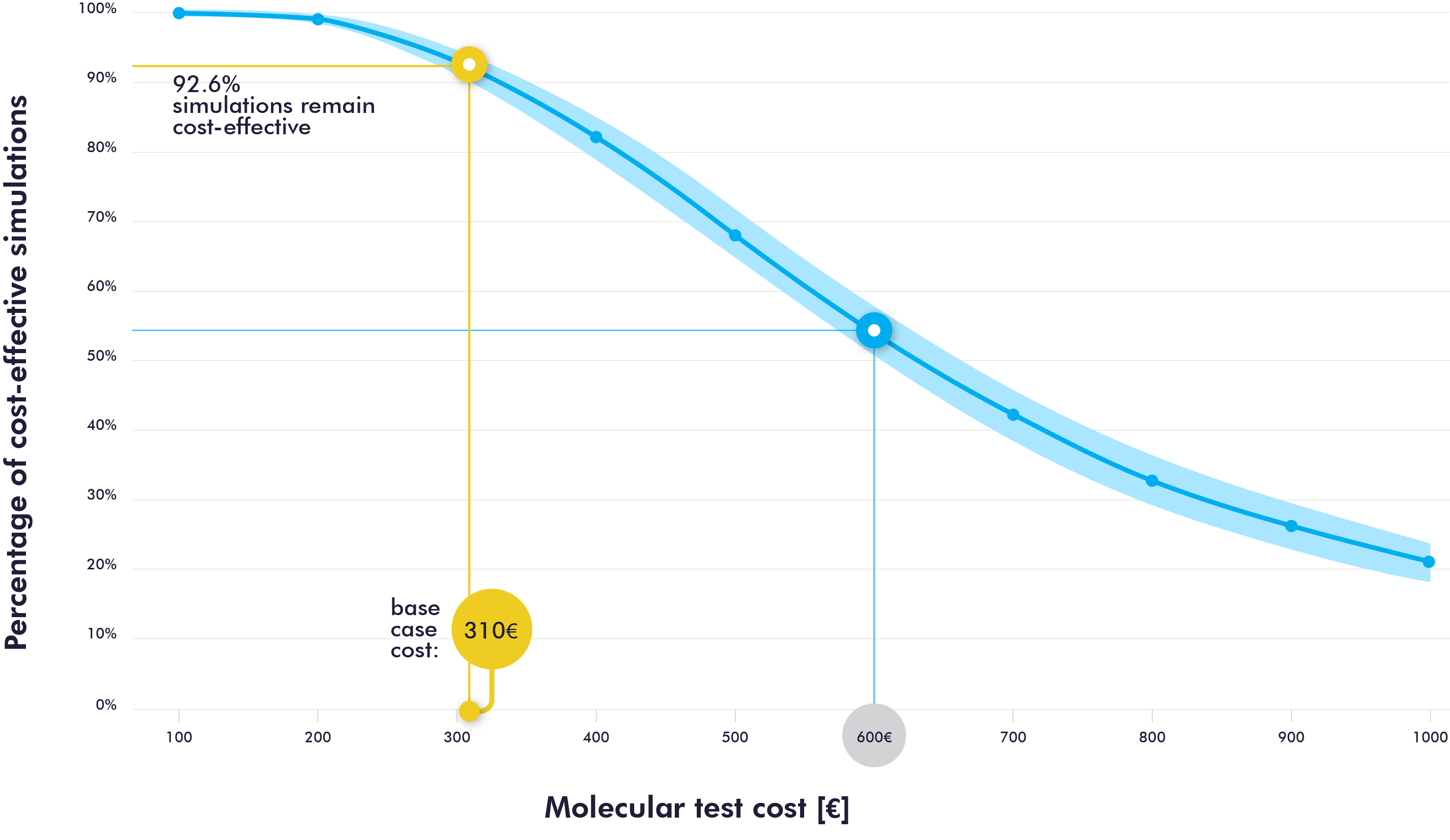
Sensitivity analyses confirmed the consistency of the results in magnitude and direction. As shown in Figure 3, the percentage of cost-effectiveness simulations for the molecular strategy is 92.6% when the molecular test cost is set at 310€. When assuming twice the cost of the baseline scenario (600€), the probability of cost-effectiveness would decrease by almost half.
Figure 3. Sensitivity analysis to assess the cost-effectiveness according to the cost of the molecular test.
The findings of this study endorse the implementation of molecular testing as a reliable tool for diagnosing endometrial cancer in women with postmenopausal bleeding. By effectively reducing the number of unnecessary hysterectomies and improving efficiency, this approach presents a promising solution for the future of endometrial cancer diagnosis.
Future Directions
Our findings provide valuable information for health decision-makers to guide resource allocation decisions, inform future policy and facilitate more effective and efficient diagnosis algorithms for endometrial cancer diagnosis. Future research should explore the feasibility of implementing the molecular strategy in clinical settings and perform a budgetary impact analysis of their introduction into the health care system. Also, it will be of interest to evaluate the long-term effects of the molecular strategy on patient outcomes and quality of life.
Selected references
- Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. European Journal of Cancer. 2018 Nov;103:356–87.
- Clarke MA, Long BJ, Del Mar Morillo A, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of Endometrial Cancer Risk With Postmenopausal Bleeding in Women: A Systematic Review and Meta-analysis. JAMA Intern Med. 2018 Sep 1;178(9):1210.
- Costas L, Frias‐Gomez J, Guardiola M, Benavente Y, Pineda M, Pavón MÁ, et al. New perspectives on screening and early detection of endometrial cancer. Int J Cancer. 2019 Jun 14;ijc.32514.
- Sroczynski G, Gogollari A, Conrads-Frank A, Hallsson LR, Pashayan N, Widschwendter M, et al. Cost-Effectiveness of Early Detection and Prevention Strategies for Endometrial Cancer—A Systematic Review. Cancers. 2020 Jul;12(7):1874.
- Havrilesky LJ, Maxwell GL, Myers ER. Cost-effectiveness analysis of annual screening strategies for endometrial cancer. American Journal of Obstetrics and Gynecology. 2009 Jun;200(6):640.e1-640.e8.
- Warring SK, Borah B, Moriarty J, Gullerud R, Lemens MA, Destephano C, et al. The cost of diagnosing endometrial cancer: Quantifying the healthcare cost of an abnormal uterine bleeding workup. Gynecologic Oncology. 2021 Oct;S0090825821015237.
- Halpern EF, Pandharipande PV. Behind the Numbers: Sensitivity Analysis in Cost-Effectiveness Modeling. Radiology. 2017 Aug;284(2):310–2.
- Briggs A. Probabilistic analysis of cost-effectiveness models: statistical representation of parameter uncertainty. Value Health. 2005 Feb;8(1):1–2.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in