Modulating the Dynamics of Brønsted Acid Sites on PtWOx Inverse Catalysts
Published in Chemistry

Motivation
Metal-metal oxide (M-MO) catalysts are central to petrochemicals, fine chemicals, pharmaceuticals, and biomass upgrade reactions1–5 owing to cooperative catalysis among multiple active sites. Inverse M-MO (M = Pt, Ir, Rh, etc.; MO = WOx, MoOx, ReOx, etc.) catalysts where the oxide is on top of a metal core have demonstrated superior activity and selectivity than single metals or oxide catalysts for converting biomass-derived molecules to high-value C3-C6 terminal diols and aromatics via ring-opening or hydrodeoxygenation.1–6 These multifunctional catalysts contain metal, redox, Brønsted, and Lewis acid sites. Establishing structure-activity relationships for these catalysts is complicated by the intimate coupling of Brønsted acidity and catalyst reducibility and their dynamic nature. Specifically, as the hydrogen availability increases for in situ Brønsted acid formation, partial reduction of the oxide also occurs, leading to redox centers (vacancy formation).7 Understanding the dynamics and coupling of multiple active sites under various reaction environments is essential to design better catalysts and processes.
Our Approach
We employ an integrated approach that combines multiscale modeling, advanced catalyst synthesis, in situ spectroscopies, reaction kinetics, and expert knowledge. We chose PtWOx/C inverse catalyst synthesized by atomic layer deposition (ALD) as a showcase. This catalyst contains a near-monolayer of WOx on the surface of Pt.
Modeling. We tested various oxide clusters on Pt(111) and found W3O9 to have a characteristic W L3 X-ray absorption near edge structure (XANES) feature similar to in situ XANES spectra acquired at the synchrotron at the Argonne National Laboratory and Brookhaven National Laboratory (Figs. 1a-d). We took W3O9 as a reasonable approximation for computational work.
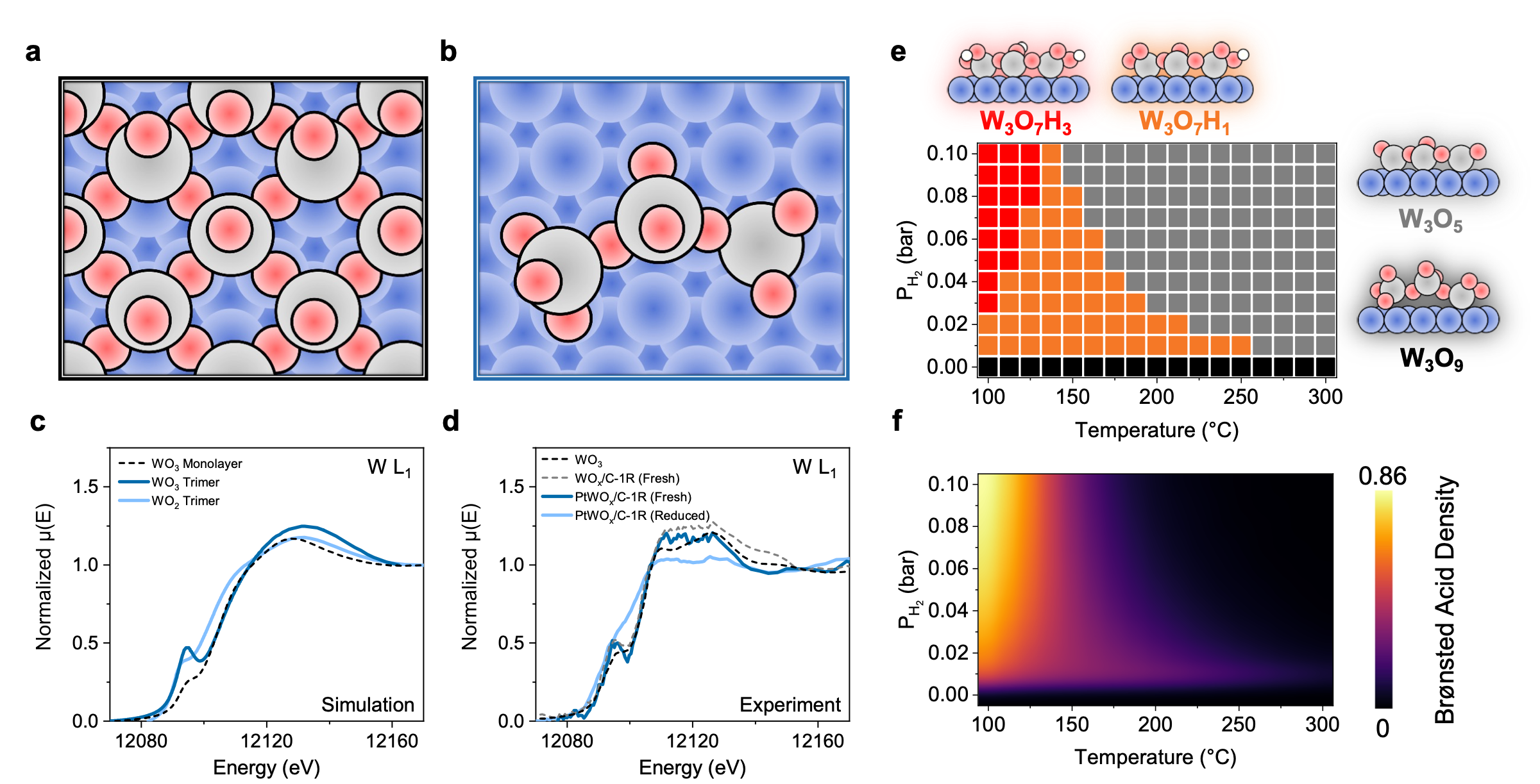
Fig. 1 | Model structures and phase behavior. a, WO3 monolayer on Pt(111). b, W3O9 cluster on Pt(111). Color code: Blue, Pt; Grey, W; Red, O. 1R indicates 1 ALD cycle. c, Simulated W L1 XANES spectra of model structures. d, Experimental W L1 XANES spectra of WO3 standard, WOx/C-1R, and PtWOx/C-1R. Reduction at 400 °C. e, ab initio phase diagram for the W3Ox/Pt(111) model. Each square represents the most abundant state under the corresponding conditions. Color code: Black square, W3O9; Gray square, W3O5; Orange square, W3O7H1; Red square, W3O7H3. f, Microkinetic heatmap of Brønsted acid site density (number of OHs per W) at long times.
We predicted the inverse-catalyst equilibrium composition of various sites and the dynamics of their formation under working conditions. Specifically, we calculated the energies for H spillover, WOx reduction, oxidation, WOx hydration, and dehydration. The model predicts that the kinetics of WOx reduction and protonation depends strongly on the oxidation state of WOx, hydrogen and water concentrations, and temperature. Ab initio phase diagrams provide the acid site densities. However, since each elementary step proceeds at a different time scale, a static model is insufficient in predicting the densities of the active sites. We developed state-based microkinetic models to capture the dynamics of inverse catalysts. Depending on the reaction environment, intermediate (non-equilibrated) states can be substantially more active than the thermodynamically stable states.
Exposing the catalyst dynamics with probe molecules. To check the robustness of our model and to probe the dynamics of Brønsted acid sites, tert-butanol dehydration was performed over the PtWOx/C inverse catalyst in a gas-phase continuous flow reactor. We found that by changing the pretreatment conditions and reaction cofeed, the activity of the inverse catalyst can be tuned by 1-2 orders of magnitude (Figs. 2a-d) and changes with time on stream. The effects of water, hydrogen, and pre-treatment temperature are in excellent qualitative agreement with our models.
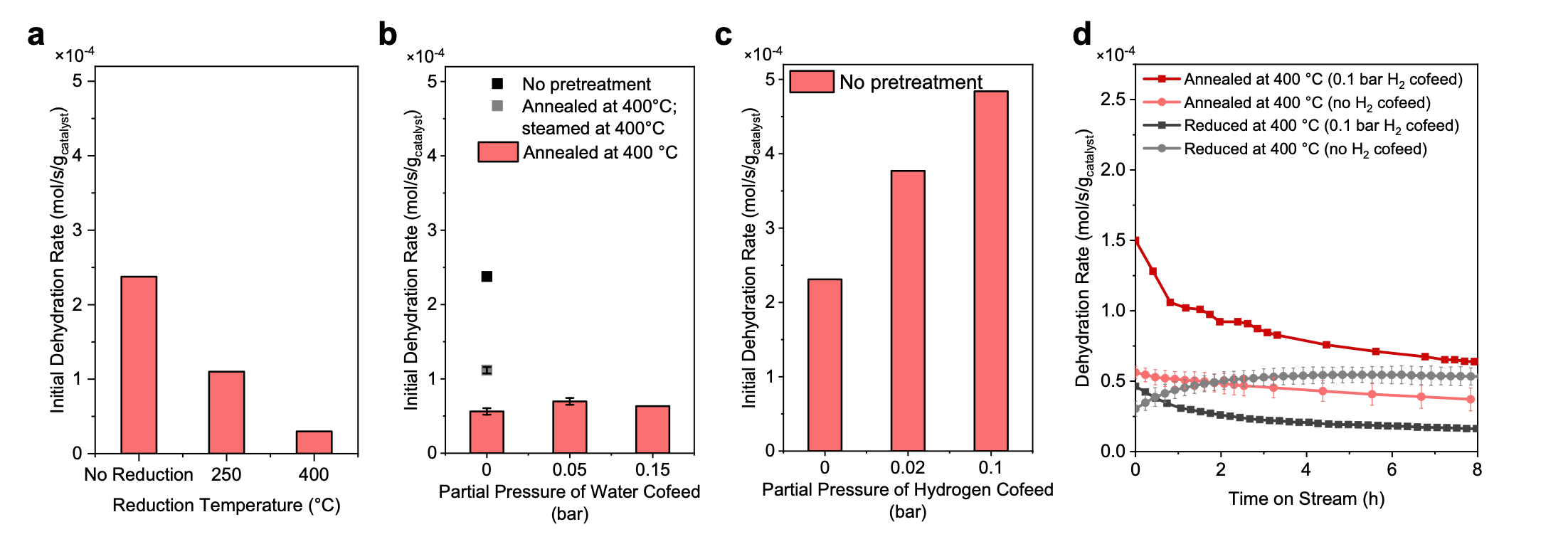
Fig. 2 | Probing active sites with alcohol probe reactions. a, reaction of tert-butanol. Catalysts reduced in 0.1 bar H2 for 1 h, carrier gas = 100 mL/min N2/He (1:1) mixture. b, modulating surface OHs with annealing and water. Annealing time = 1 h, steaming time = 1 h with water partial pressure = 0.1 bar. c, effect of hydrogen partial pressure on the dehydration activity of fresh PtWOx/C-1R. d, effect of oxidation state on the in situ formation of Brønsted acid sites by hydrogen. Reaction conditions for (a)-(d): 140 °C, 4 mol% tert-butanol, 10 mg catalyst. The dehydration rate accounts for the sum of isobutene and isobutane.
Tuning activity by periodic pulsing of H2. Inspired by the modeling and probe chemistry insights, we designed a periodic H2 pulsing strategy to increase the reaction rate by leveraging the dynamics of WOx (Fig. 3a). Fig. 3b shows time on stream data for a reduced catalyst. Periodic H2 pulsing allows the catalyst to reach alternate states with a much higher acid site density. The average dehydration rate over the last 4 h is about one order of magnitude higher than that with a constant hydrogen cofeed (Fig. 3c). One may expect more than one order of magnitude increase in catalyst activity upon optimizing the frequency of periodic pulsing.
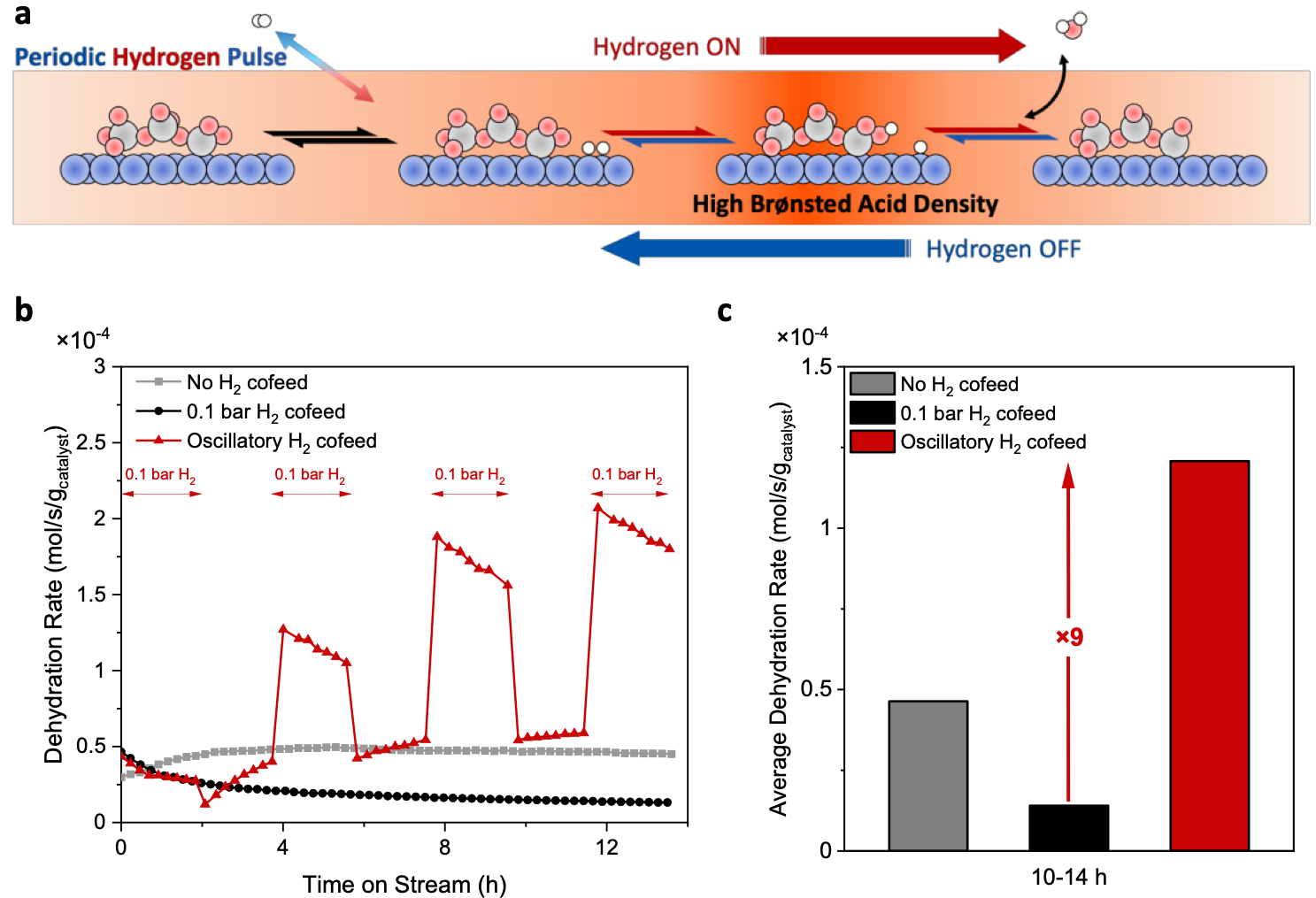
Fig. 3 | Impact of hydrogen pulsing on reaction rate. a, Schematic of periodic hydrogen pulse for sustaining high Brønsted acid site density. b, Time on stream data of the hydrogen pulse cofeed experiment. PtWOx/C-1R is pre-reduced at 400 °C in 0.1 bar H2 for 1 h prior to reaction. Reaction conditions: 140 °C, 4 mol% tert-butanol, 10 mg catalyst, carrier gas = 100 mL/min H2/N2 mixture (0.1 bar H2) or N2. c, Average dehydration rates from 10 h to 14 h.
Outlook
Our work demonstrates the power of integrating multiscale modeling, reaction kinetics, and in situ characterization to understand complex catalyst dynamics under reaction conditions. It paves a viable way for controlling this intriguing and important class of catalysts. We envision that catalyst performance enhancement would be achievable over complex core-shell M-MO catalysts for many other key reactions.
References
- Védrine, J. C. Heterogeneous catalysis on metal oxides. Catalysts 7, 341 (2017).
- Tomishige, K., Nakagawa, Y. & Tamura, M. Selective hydrogenolysis and hydrogenation using metal catalysts directly modified with metal oxide species. Green Chem. 19, 2876–2924 (2017).
- Chia, M. et al. Selective hydrogenolysis of polyols and cyclic ethers over bifunctional surface sites on rhodium-rhenium catalysts. J. Am. Chem. Soc. 133, 12675–12689 (2011).
- Wang, C. et al. Mechanistic Study of the Direct Hydrodeoxygenation of m-Cresol over WOx-Decorated Pt/C Catalysts. ACS Catal. 8, 7749–7759 (2018).
- Wang, C. et al. A Study of Tetrahydrofurfuryl Alcohol to 1,5-Pentanediol Over Pt – WO x / C. Catal. Letters 148, 1047–1054 (2018).
- Fu, J. et al. C-O bond activation using ultralow loading of noble metal catalysts on moderately reducible oxides. Nat. Catal. 3, 446-453 (2020).
- Rasmussen, M. J. & Medlin, J. W. Role of tungsten modifiers in bimetallic catalysts for enhanced hydrodeoxygenation activity and selectivity. Catal. Sci. Technol. 10, 414–423 (2020).
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in