Moisture-resistant Nanolaminate Membranes Enabled by Charge Neutralizing Nanofillers
Published in Electrical & Electronic Engineering

While being an essential atmospheric agent for sustaining a wide range of life forms, moisture can often be a sticky issue for industrial products and productions. We, therefore, try to protect healthcare products, food, electronics, and even textiles from humidity using waterproof coatings, packaging materials, and dehumidifiers, for example. But when it comes to the chemical processes that create humidity as a by-product, it is rather hard to deal with its complications sometimes. Hydrogen (H2) production is one such example.
Several scalable methodologies, such as water electrolysis and fossil fuel reforming, can produce large volumes of H2; but the product is never pure. In the case of water electrolysis, H2 should be separated from oxygen (O2). Fossil fuel conversions, on the other hand, bring several major contaminants, including carbon dioxide (CO2). And, in either case, humidity is inescapable, adding up to the list of substances to remove. Plus, humidity usually complicates the removal of other components from gas mixtures.
To separate a mixture of substances, one can pick different options among a plethora of tools. The bottom line is that the selected separation strategy should provide desired purity at a reasonable economic and environmental cost. Membrane separation systems are, thus, appealing as they do not require additional chemicals or phase change. Nevertheless, only well-optimized membrane materials give high separation efficiency and ensure operational stability.
Our research group at Kyoto University actively explores new materials for developing membranes with superior performances to address various molecular separation needs with direct relevance to the energy sector and environmental issues. And we strive to test the limits of new membrane materials under more realistic settings, considering the impact of existing or envisioned process conditions. The same applies to our efforts to use two-dimensional (2D) materials, which are relatively new in membrane development.
Following the isolation of graphene from graphite in 2004 [1], we witnessed a growing interest in exploiting 2D materials for gas and liquid separations [2][3]. Atomic-level thicknesses and micrometer-level lateral sizes of graphene-based materials enable designing advanced membranes with superlow transport resistances and superhigh permeation fluxes, making them fundamentally different from their ordinary counterparts. The oxidized and exfoliated form of graphite, graphene oxide (GO), is particularly advantageous owing to its high processibility originating from water solubility. However, as we observed first-hand in the early stages of our research on GO-based laminates, water-solubility also causes operational instability issues. When exposed to water molecules, the distances between the individual GO layers typically expand, deteriorating the membrane structure in time. This “swelling effect” is governed by the hydration of oxygen-containing groups and favors the charge-charge repulsion of negatively charged GO materials.
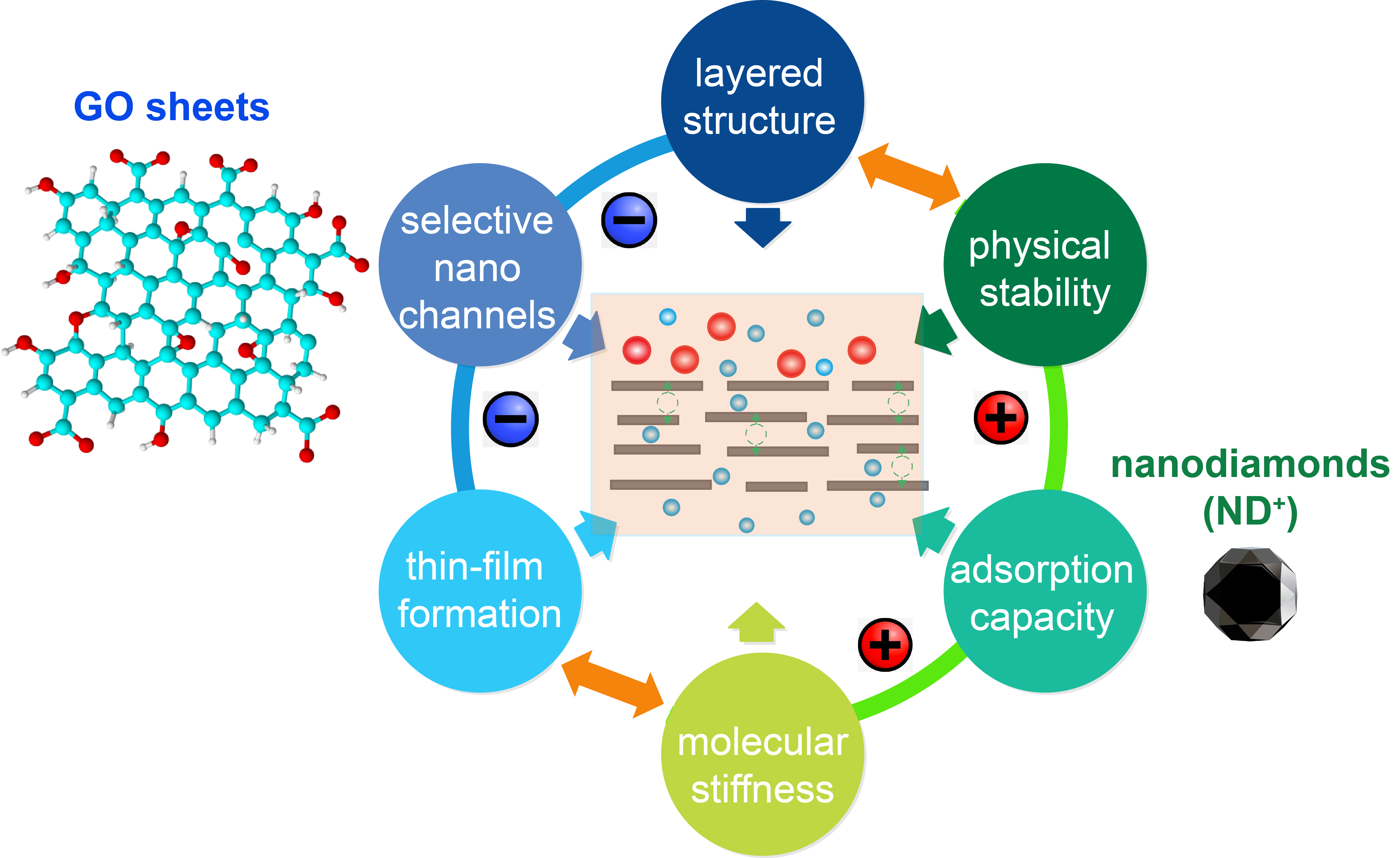
Figure 1. The strategy proposed in this work for developing hybrid carbon nanoarchitectures as molecular sieving membrane materials. Credit: Behnam Ghalei and H. Enis Karahan.
In our recent paper published in Nature Energy, we explicitly aimed at stabilizing GO membranes against undesirable humidity effects while maintaining high H2 purification performance. To do so, taking the compatibility of carbonaceous materials into account, we combined positively charged nanodiamonds (ND+) with negatively charged GO nanosheets (Figure 1). The electrostatic interactions between ND+ and GO surfaces allowed us to prepare robust ultrastructures that operate well under humid gas mixtures (Figure 2).
In addition to enhancing water stability, ND+ particles also increased the overall pore volume and reduced the size of well-stacked GO domains. Therefore, the incorporation of ND+ particles into GO nanolaminates manipulated the pore architecture, making the resulting membranes (GOαND+) more permeable, However, GOαND+ membranes could still retain the H2/CO2 selectivity of GO (for dry gas mixture) significantly even under humid conditions. Thus, overall, we consider the ND+ incorporation a one-stone-two-birds strategy.
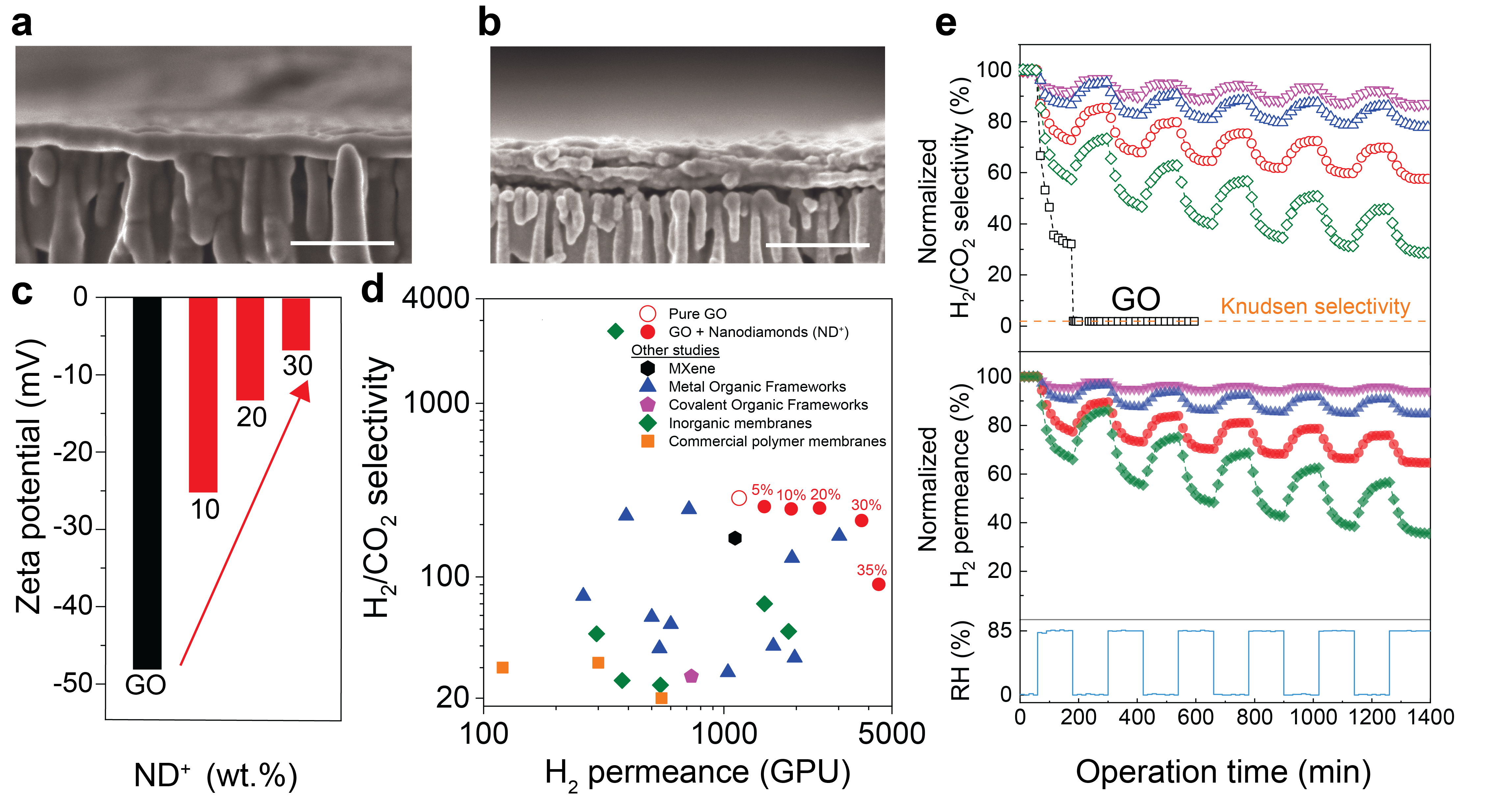
Figure 2. a, b. Cross-sectional (scale bar: 200 nm) FESEM images of vacuum-filtered GO membranes and GO30ND+ membranes. c. The zeta potential values of GO (black bar), GOαND+ (red bars) composites at different loadings (pH 7). d. H2/CO2 separation performance of the membranes developed in this work (red circles, the numbers indicate the ND+ content in the membranes) compared to the state-of-the-art H2 separation. e. The normalized H2/CO2 separation factors (open symbols) and the normalized H2 permeance (solid symbols) of the GO (black), GO5ND+ (green), GO10ND+ (red), GO20ND+ (blue) and GO30ND+ (purple) membranes under six continuous humid (85% RH)/dry (0% RH) cycles and equimolar H2/CO2 mixed-gas feed. (These figures are taken from our publication as-is or in a simplified manner.)
Furthermore, the water-resistant laminated nanocomposites delivered in this work are also of interest beyond membrane separation, including energy-related and other environmental applications, such as microsupercapacitors, fuel cells, and sensors.
For more details, please see the original version of the manuscript:
www.nature.com/articles/s41560-021-00946-y
Acknowledgments
The author thanks Dr. H. Enis Karahan and Prof. Easan Sivaniah for fruitful discussions and Dr. H. Enis Karahan for text revision.
References
[1] K.S. Novoselov, A.K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva, A.A. Firsov, Electric field in atomically thin carbon films, Science. 306 (2004) 666–669. doi:10.1126/science.1102896.
[2] S.P. Koenig, L. Wang, J. Pellegrino, J.S. Bunch, Selective molecular sieving through porous graphene, Nat. Nanotechnol. 7 (2012) 728–732. doi:10.1038/nnano.2012.162.
[3] G. Liu, W. Jin, N. Xu, Graphene-based membranes, Chem. Soc. Rev. 44 (2015) 5016–5030. doi:10.1039/C4CS00423J.
[4] K.H. Thebo, X. Qian, Q. Zhang, L. Chen, H.-M. Cheng, W. Ren, Highly stable graphene-oxide-based membranes with superior permeability, Nat. Commun. 9 (2018) 1486. doi:10.1038/s41467-018-03919-0.
Follow the Topic
-
Nature Energy

Publishing monthly, this journal is dedicated to exploring all aspects of this on-going discussion, from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
Related Collections
With Collections, you can get published faster and increase your visibility.
Microgrids and Distributed Energy Systems
Publishing Model: Hybrid
Deadline: Mar 31, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in